Evaluating the impact of various aspects on the functionality of poly (methacrylic acid-co-ethyl acrylate)
Various active pharmaceutical ingredients (APIs) are either aggressive to the stomach’s mucosa or vulnerable to the acidic nature of the gastric juice. Solid oral dosage forms carrying one of these APIs thence require gastric resistant functionality. Poly(methacrylic acid-co-ethyl acrylate) [MAE] based coats are most frequently applied to introduce gastric resistant functionality to a solid oral dosage form.
The polymer is available in three different grades (Ph.Eur.):
- an aqueous dispersion:
Methacrylic acid – ethyl acrylate copolymer (1:1) dispersion 30 per cent (e.g. Kollicoat® MAE 30 DP), - a powder grade (non-partly pre-neutralised):
Methacrylic acid – ethyl acrylate copolymer (1:1), Type A (e.g. Kollicoat® MAE 100-55) - a powder grade (partly pre-neutralised [6 mol%]):
Methacrylic acid – ethyl acrylate copolymer (1:1), Type B (Kollicoat® MAE 100 P)
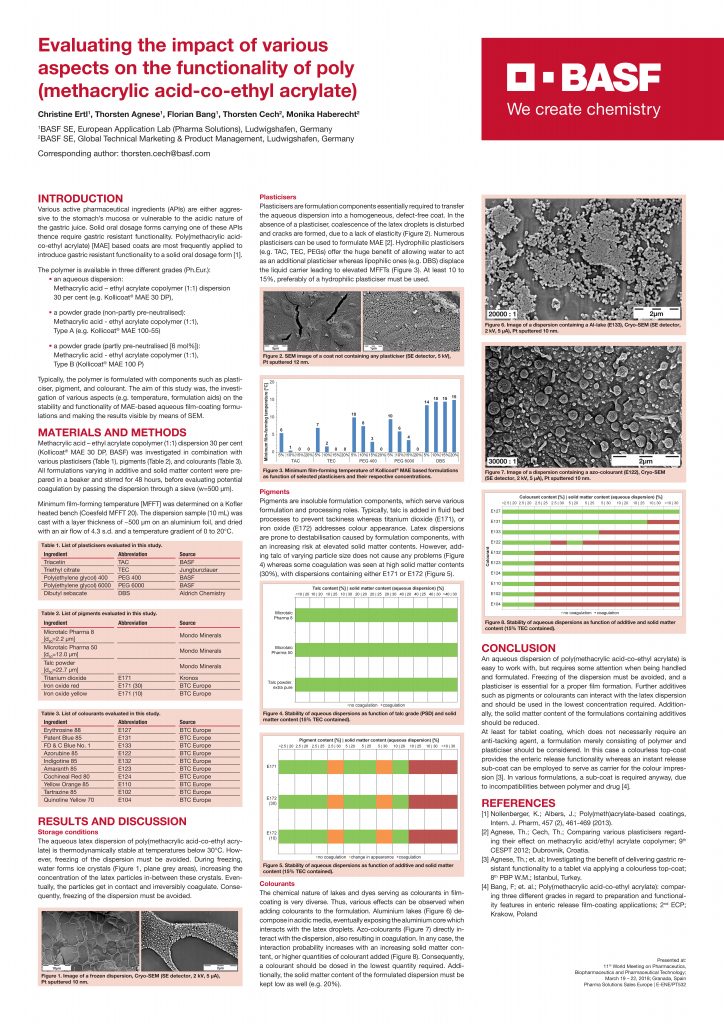
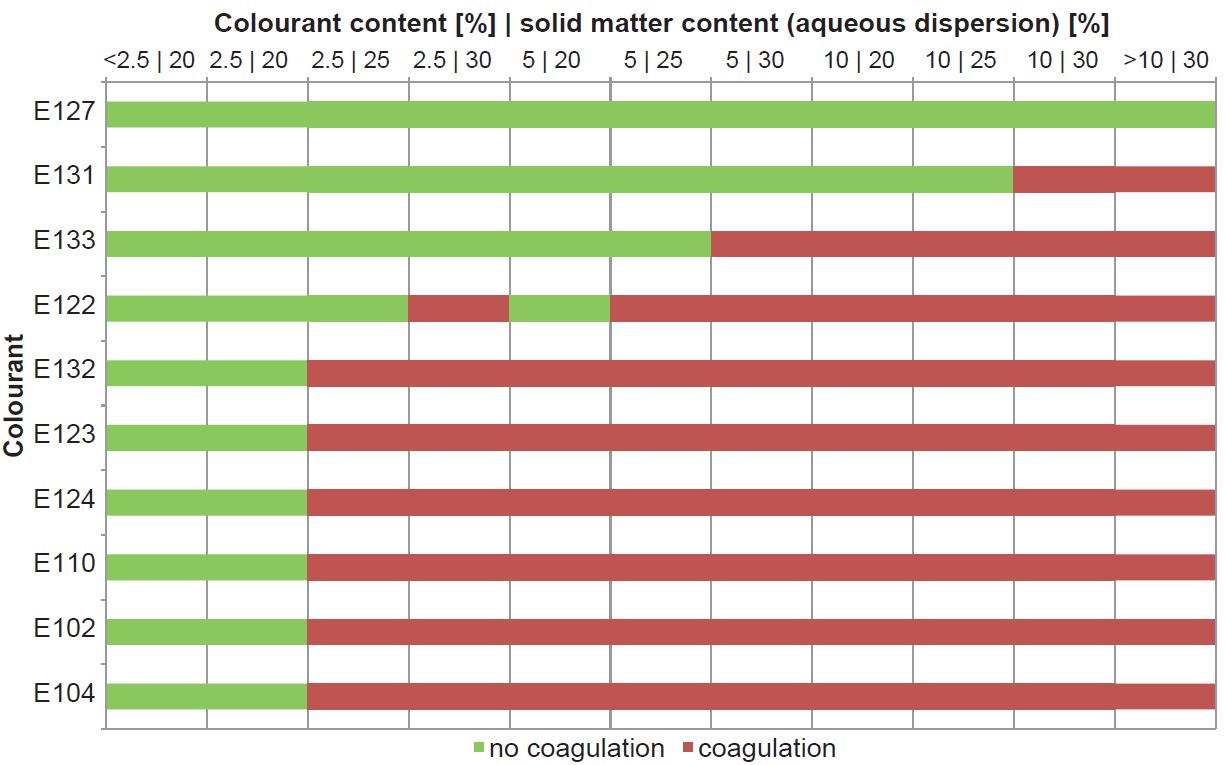
Typically, the polymer is formulated with components such as plasticiser, pigment, and colourant. The aim of this study was, the investigation of various aspects (e.g. temperature, formulation aids) on the stability and functionality of MAE-based aqueous film-coating formulations and making the results visible by means of SEM.
Click poster image to enlarge:
See also our interview with Dr. Ferdinand Brandl from BASF on ZoomLab™. Dr. Brandl answered our questions on the idea and start of ZoomLab™. He also gave us an overview on the next modules and the development in the next year. See our interview here.