Exploring the Interplay between Drug Release and Targeting of Lipid-Like Polymer Nanoparticles Loaded with Doxorubicin
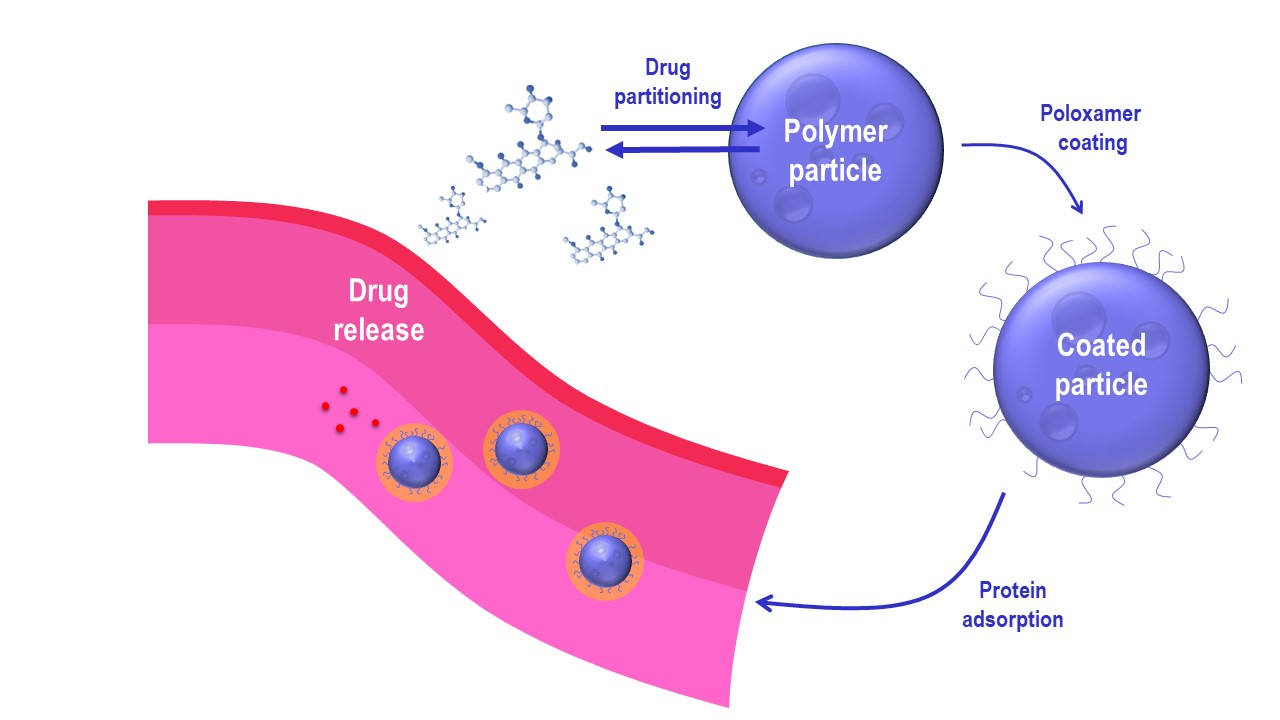
Targeted delivery of doxorubicin still poses a challenge with regards to the quantities reaching the target site as well as the specificity of the uptake. In the present approach, two colloidal nanocarrier systems, NanoCore-6.4 and NanoCore-7.4, loaded with doxorubicin and characterized by different drug release behaviors were evaluated in vitro and in vivo. The nanoparticles utilize a specific surface design to modulate the lipid corona by attracting blood-borne apolipoproteins involved in the endogenous transport of chylomicrons across the blood–brain barrier. When applying this strategy, the fine balance between drug release and carrier accumulation is responsible for targeted delivery. Drug release experiments in an aqueous medium resulted in a difference in drug release of approximately 20%, while a 10% difference was found in human serum.
This difference affected the partitioning of doxorubicin in human blood and was reflected by the outcome of the pharmacokinetic study in rats. For the fast-releasing formulation NanoCore-6.4, the AUC0→1h was significantly lower (2999.1 ng × h/mL) than the one of NanoCore-7.4 (3589.5 ng × h/mL). A compartmental analysis using the physiologically-based nanocarrier biopharmaceutics model indicated a significant difference in the release behavior and targeting capability. A fraction of approximately 7.310–7.615% of NanoCore-7.4 was available for drug targeting, while for NanoCore-6.4 only 5.740–6.057% of the injected doxorubicin was accumulated. Although the targeting capabilities indicate bioequivalent behavior, they provide evidence for the quality-by-design approach followed in formulation development.
Download the full article here: Exploring the Interplay between Drug Release and Targeting of Lipid-Like Polymer Nanoparticles Loaded with Doxorubicin
or continue reading here: Kovshova, T.; Osipova, N.; Alekseeva, A.; Malinovskaya, J.; Belov, A.; Budko, A.; Pavlova, G.; Maksimenko, O.; Nagpal, S.; Braner, S.; Modh, H.; Balabanyan, V.; Wacker, M.G.; Gelperina, S. Exploring the Interplay between Drug Release and Targeting of Lipid-Like Polymer Nanoparticles Loaded with Doxorubicin. Molecules 2021, 26, 831. https://doi.org/10.3390/molecules26040831

