Evaluation of polymer choice on immunogenicity of chitosan coated PLGA NPs with surface-adsorbed pneumococcal protein antigen PspA4Pro
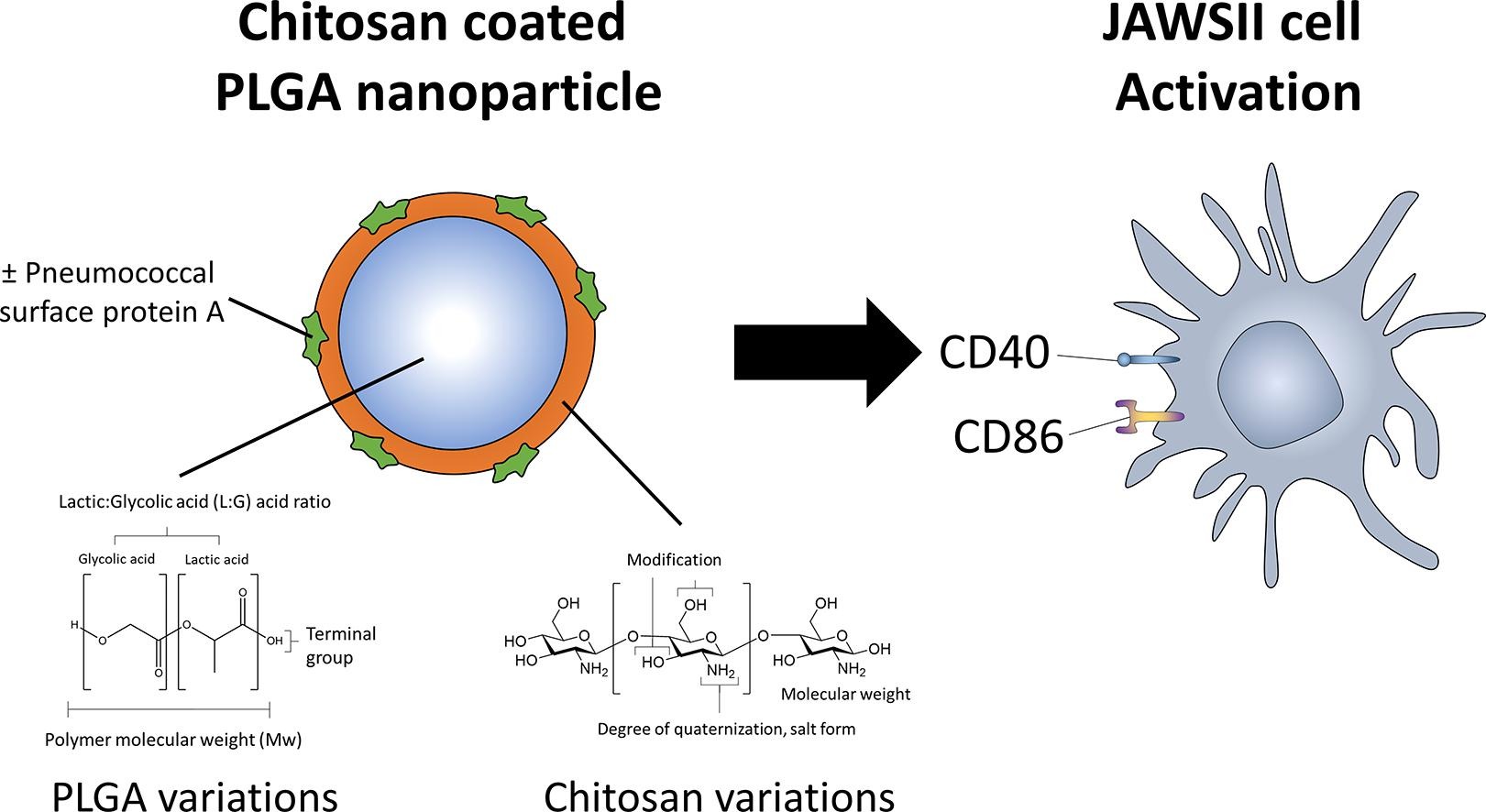
Polymeric nanoparticles (NPs) are recognized as potential delivery vehicles for vaccines. PLGA is a biocompatible polymer synonymous with polymeric NPs, which can be coated with other polymers such as chitosan that has intrinsic adjuvant properties as well as mucoadhesive properties. Numerous modifications and variations exist for PLGA and chitosan, which can influence the NP characteristics and the resulting immunogenicity. The current study investigated variations for making chitosan coated PLGA NPs incorporating recombinant pneumococcal surface protein A from family 2, clade 4 (PspA4Pro) antigen as a vaccine targeting the vast majority of pneumococcal strains and determine the effect of the polymers on particle size, surface charge, and surface marker upregulation on a dendritic cell (DC) line in vitro.
PLGA variations tested with the ester-terminal group had the greatest detriment for prospective vaccine use, due to the lowest PspA4Pro adsorption and induction of CD40 and CD86 cell surface markers on DCs. The negatively charged chitosans exhibited the lowest surface marker expressions, similar to the uncoated NP, supporting the commonly accepted notion that positive surface charge augments immunogenic effects of the NPs. However, the study indicated that NPs made from PLGA with an acid terminated group, and chitosan HCl salt, exhibit particle characteristics, antigen adsorption efficiency and immunogenicity, which could be most suitable as a vaccine formulation.
Download the full research paper as pdf: Evaluation of polymer choice on immunogenicity of chitosan coated PLGA NPs with surface-adsorbed pneumococcal protein antigen PspA4Pro
Materials:
PLGA (Lactide:glycolide (50:50), acid terminated, average Mw 7–17 kDa), (Lactide:glycolide (50:50), ester terminated, average Mw 7–17 kDa), (Lactide:glycolide (50:50), acid terminated, average Mw 24–38 kDa) and (Lactide:glycolide (75:25), acid terminated, average Mw 4–15 kDa) polyvinyl alcohol (PVA), were obtained from Sigma, UK. Chitosan hydrochloride (HCl), carboxymethyl chitosan (CMC), chitosan oligomer, chitosan glutamate, were obtained from Heppe Medical Chitosan GmbH, Germany.
Kan Kaneko, Eliane N. Miyaji, Viviane M. Gonçalves, Daniela M. Ferreira, Carla Solórzano, Ronan MacLoughlin, Imran Saleem,
Evaluation of polymer choice on immunogenicity of chitosan coated PLGA NPs with surface-adsorbed pneumococcal protein antigen PspA4Pro, International Journal of Pharmaceutics, Volume 599, 2021,120407,
https://doi.org/10.1016/j.ijpharm.2021.120407.
See also our article:


