Oral delivery of peptide therapeutics in infants: Challenges and opportunities
Abstract: The vast majority of drugs are not designed or developed for pediatric and infant populations. Peptide drugs, which have become increasingly relevant in the past several decades, are no exception. Unfortunately, nearly all of the 60+ approved peptide drugs are formulated for injection, a particularly unfriendly mode of administration for infants. Although three peptide drugs were recently approved for oral formulations, this major advance in peptide drug delivery is available only for adults. In this review, we consider the current challenges and opportunities for the oral formulation of peptide therapeutics, specifically for infant populations. We describe the strategies that enable oral protein delivery and the potential impact of infant physiology on those strategies. We also detail the limited but encouraging progress towards 1) adapting conventional drug development and delivery approaches to infants and 2) designing novel infant-centric formulations. Together, these efforts underscore the feasibility of oral peptide delivery in infants and provide motivation to increase attention paid to this underserved area of drug delivery and formulation.
Download the full article here or read it here
Introduction
Historically, drugs are not designed for children or infants. This is because children, as a population, present many challenges from a drug development perspective. For example, there are ethical concerns when testing medications in children and financial motivation is insufficient. Furthermore, children have unique needs in terms of drug dosage forms as well as physiology that is fundamentally different than that of adults.
These drug development challenges result in two major problems. The first is that medication simply is not available for many infant and childhood conditions, including necrotizing enterocolitis, childhood interstitial lung disease, and autosomal recessive polycystic kidney disease [1], [2], [3]. This, in turn, may account for the 10-fold lower enrollment of pediatric patients in clinical trials compared to adults [4]. The second problem is that when an appropriate medication does exist, it is often an adult medication that has been prescribed “off-label”. Such use can entail altering the approved dose, time period, dosage form, and/or route of administration. Unfortunately, off-label prescriptions lead to twice as many adverse drug reactions (e.g. vomiting, seizures) as licensed drugs [5], [6] and are associated with increased patient mortality, given the poorly understood physiological differences between adults and children [7]. Furthermore, dose adjustments are typically performed by educated guesswork based on weight or body surface area. Thus, there is a clear need for optimized drug formulations designed specifically for pediatric populations.
The oral route of drug delivery is considered to be the most patient-preferred because it results in the highest levels of compliance [8]. Oral formulations are easily administered outside of healthcare settings, ideal for longer-term use, and can be readily discontinued in the event of adverse reactions. Furthermore, tablets and other solid dosage forms facilitate longer shelf lives of the active pharmaceutical ingredients (API) compared to sterile and specialty pharmaceutical dosage forms [9]. Pediatric patients, in particular, benefit from oral medications because children tend to be more distressed by injections than adults. Although children sometimes have difficulty swallowing solid dosage forms, a number of antibiotics and non-steroidal anti-inflammatory drugs have been formulated as liquids to ease administration to pediatric patients [10]. Because very few drugs are formulated specifically for infants, pharmacists often extemporaneously dissolve, dilute, or resuspend formulations made for adults at different concentrations or in different media for infant use [11], [12]. This is a major safety concern, and fatal mistakes have been made due to the complexity of altering adult formulations with no clear guidance or standards [13].
Peptide and protein drugs are a unique and critical sector of the pharmaceutical market, with three new peptide drugs approved in 2019-2020 and an additional 150 in active development [14], [15]. Peptide drugs have a number of key advantages over small molecule drugs, including greater specificity and more sophisticated pharmacological mechanisms of action, which can be exploited for treating more complex diseases. Successful peptide drugs include insulin for the treatment of diabetes, and human growth hormone for treating growth disorders. However, peptide therapeutics suffer from the major disadvantage that they are difficult to formulate and deliver, particularly via the oral route, due to their physicochemical characteristics. Because these challenges have delayed the development and market approval of peptide drugs, they have also hindered the implementation of these drugs in pediatric populations.
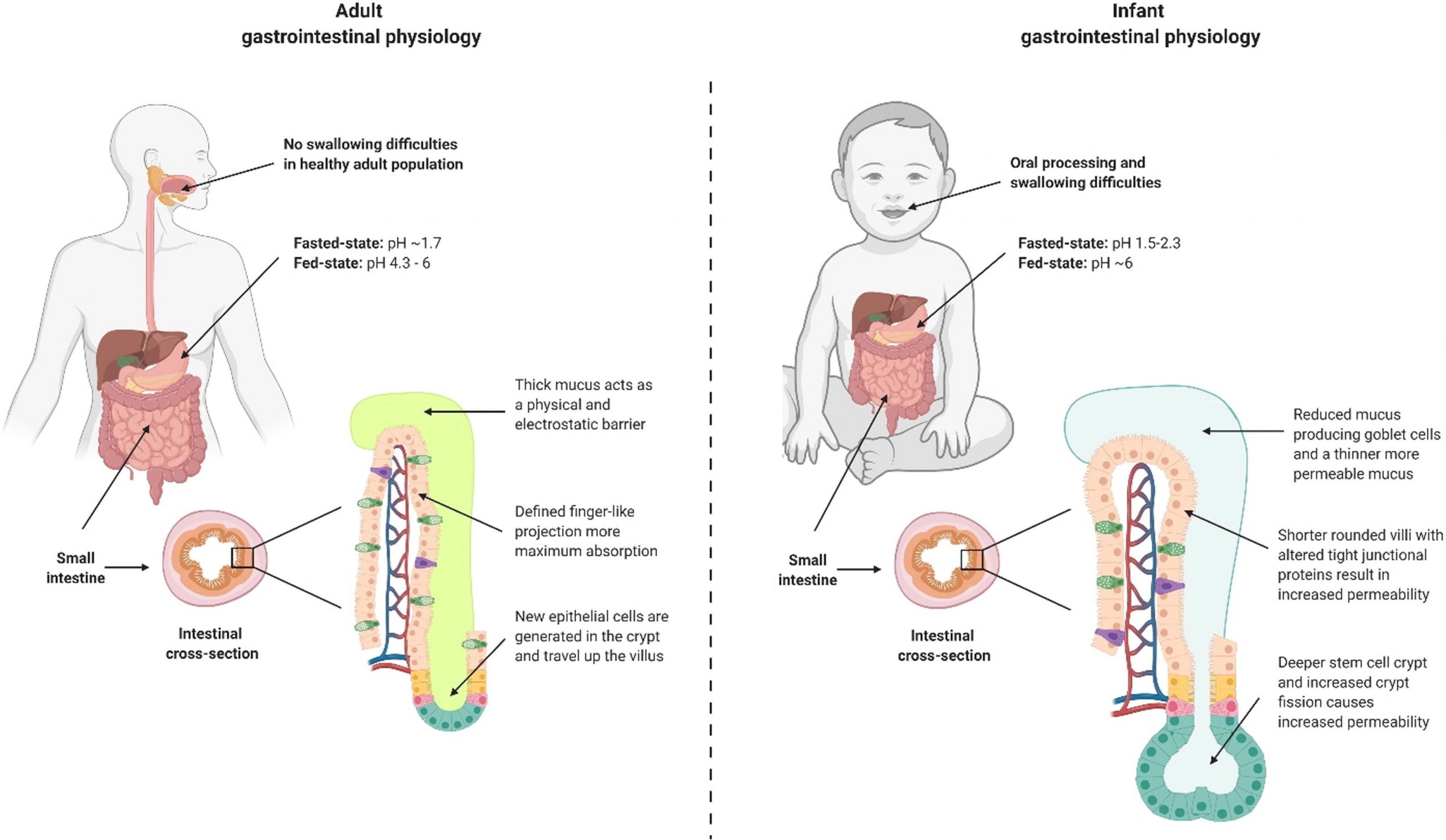
In this review, we consider the current challenges and opportunities for the oral formulation of peptide therapeutics, specifically for infant populations. Infants are defined as being between 1 and 24 months of age [16]. An alternative review is available on drug delivery for neonates, which are babies less than one month of age [11]. In the first half of the review, we discuss the current status of the peptide drug pipeline for infants, and the challenges and solutions for oral formulation of peptide drugs. The second part of the review focuses on the distinct infant and adult gastrointestinal physiology that poses unique drug delivery challenges, highlighting the critical gaps of knowledge in this area. We also present the encouraging progress towards adapting conventional drug development and delivery approaches to infants.
Download the full article here or read it here
Article information: John P. Gleeson, Katherine C. Fein, Kathryn A. Whitehead. Oral delivery of peptide therapeutics in infants: Challenges and opportunities, Advanced Drug Delivery Reviews, 2021. https://doi.org/10.1016/j.addr.2021.03.011.


