Review: Continuous Manufacturing of Small Molecule Solid Oral Dosage Forms

Introduction
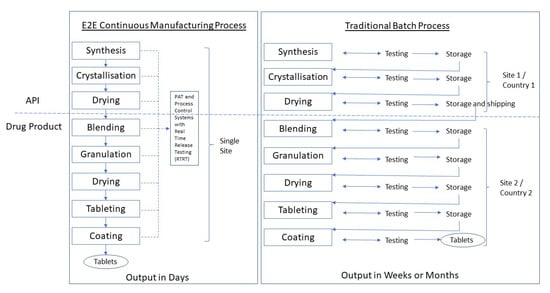
Note: API manufacturing has traditionally occurred at a primary manufacturing site typically separate from the drug product site and often in a different (low-tax) country.
Regulatory authorities (EMA, MHRA, FDA and PDMA) have all been strong advocates of CM in particular for its benefits to improve the quality and control of drug products and to produce a more flexible supply chain. The industry has been slow to adopt the approach mainly due to its conservatism, the cost implications and the potential difficulty of registering CM products. Regulators have suggested that CM is suited to NCEs requiring new facilities or to established products with expanding markets. Most investment in CM has been made by Big Pharma working with some academic and not-for-profit centres. There is limited information on CDMO (Contract Development and Manufacturing Organisations) involvement in CM, although Patheon (Thermo Fisher Scientific), Catalent and Aesica have been mentioned as having CM capabilities. The drivers for generic companies to be involved are different to those of pharma companies, and the initial investment and technical requirements for data handling, etc. make their involvement less likely. A model where there are hubs between academic centres and CDMOs to develop CM processes may be the way forward. Continue reading the review on continuous manufacturing

