Development of a novel beta-glucan supplemented hydrogel spray formulation and wound healing efficacy in a db/db diabetic mouse model
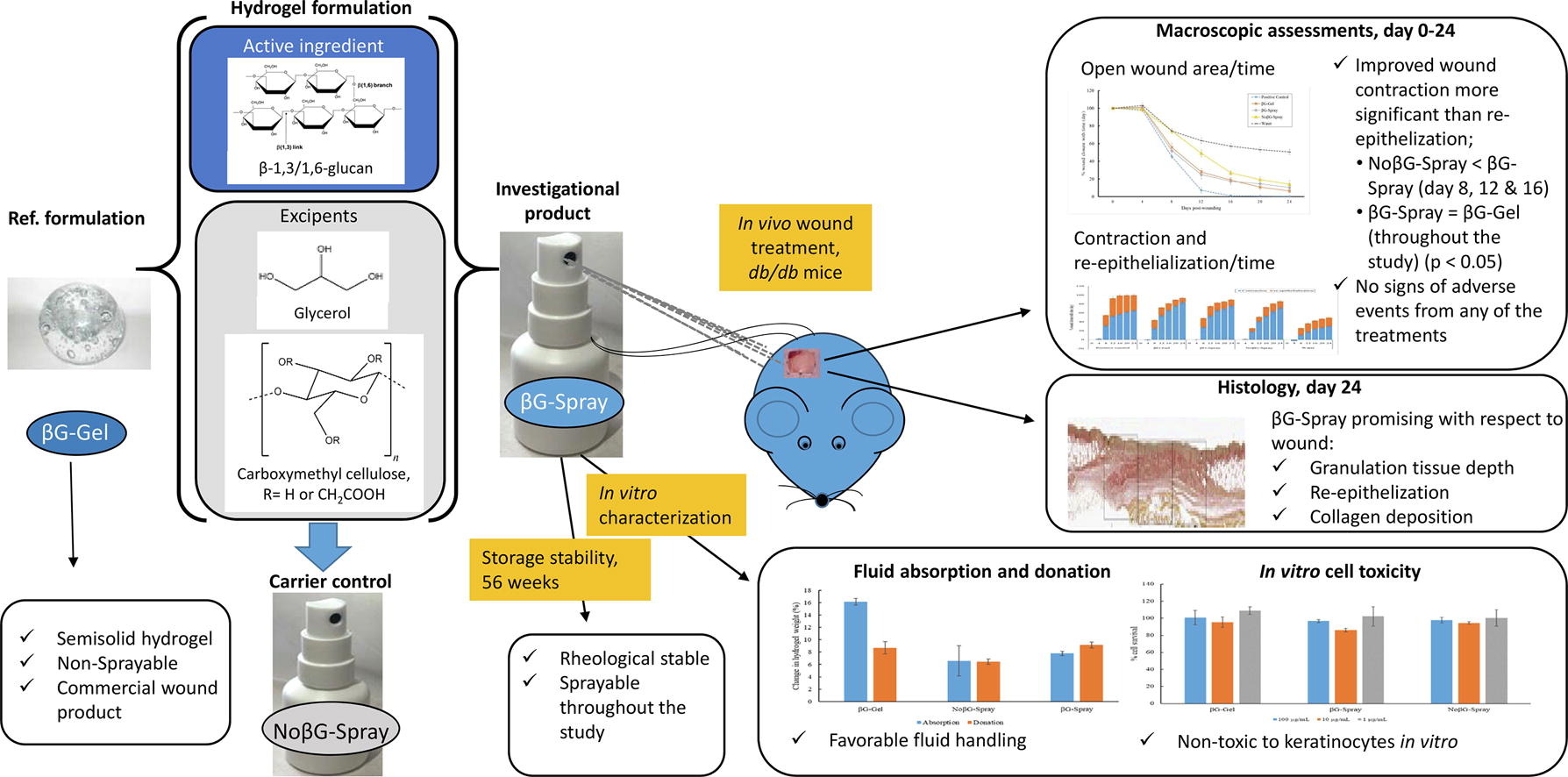
To relief the severe economic and social burdens and patient suffering caused by the increasing incidence of chronic wounds, more effective treatments are urgently needed. In this study, we focused on developing a novel sprayable wound dressing with the active ingredient β-1,3/1,6-glucan (βG). Since βG is already available as the active ingredient in a commercial wound healing product provided as a hydrogel in a tube (βG-Gel), the sprayable format should bring clinical benefit by being easily sprayed onto wounds; whilst retaining βG-Gel’s physical stability, biological safety and wound healing efficacy. Potentially sprayable βG hydrogels were therefore formulated, based on an experimental design setup. One spray formulation, named βG-Spray, was selected for further investigation, as it showed favorable rheological and spraying properties.
The βG-Spray was furthermore found to be stable at room temperature for more than a year, retaining its rheological properties and sprayability. The cytotoxicity of βG-Spray in keratinocytes in vitro, was shown to be promising even at the highest tested concentration of 100 μg/ml. The βG-Spray also displayed favorable fluid affinity characteristics, with a capacity to both donate and absorb close to 10% fluid relative to its own weight.
Finally, the βG-Spray was proven comparably effective to the commercial product, βG-Gel, and superior to both the water and the carrier controls (NoβG-Spray), in terms of its ability to promote wound healing in healing-impaired animals. Contraction was found to be the main wound closure mechanism responsible for the improvement seen in the βG-treatment groups (βG-Spray and βG-Gel). In conclusion, the novel sprayable βG formulation, confirmed its potential to expand the clinical use of βG as wound dressing.
Download the full research article as PDF here: Development of a novel beta-glucan supplemented hydrogel spray formulation and wound healing efficacy in a db-db diabetic mouse model
2.1. Materials
Glycerol (1, 2, 3-propanetriol) was purchased from VWR (Fontenay sous Bois, France). Milli-Q water was produced using a Direct 8 Water Purification System by Merck Millipore (Billerica, MA, USA). Soluble β-1,3/1,6-glucan (βG; 2.5 % w/w) and Woulgan® Gel (βG-Gel) were gifted by Biotec Betaglucans AS (Tromsø, Norway). Sodium carboxymethyl cellulose (CMC) 7M1F (MW 250,000) was purchased from Ashland (Wilmington, DE, USA). Gelatin from porcine skin was obtained from Sigma-Aldrich (Taufkirchen, Germany) and Acto™ Agar was purchased from BD (Le Pont de Claix, France). The HaCaT cell line (immortalized human keratinocytes) was purchased from Thermo Fisher Scientific (Waltham, USA). RPMI growth medium was obtained from Sigma Aldrich (Steinheim, Germany). The MTT cell proliferation kit assay was purchased from Roche (Sigma Aldrich). The rh-platelet-derived growth factor-BB (PDGF-BB) and rh-transforming growth factor-α (TGF-α) were purchased from PeproTech EC Ltd (London, UK). Isoflurane (IsoFlo®) was from Zoetis (London, UK), and Buprenorphine (Vetergesic®) was purchased from Alstoe Animal Health (Espoo, Finland). 10% Neutral Buffered Formalin, Haematoxylin and Eosin were purchased from Sigma. Picrosirius red solution was purchased from Pioneer Research Chemicals (UK).
Jostein Grip, Erik Steene, Rolf Einar Engstad, Jeff Hart, Andrea Bell, Ingrid Skjæveland, Purusotam Basnet, Nataša Škalko-Basnet, Ann Mari Holsæter, Development of a novel beta-glucan supplemented hydrogel spray formulation and wound healing efficacy in a db/db diabetic mouse model, European Journal of Pharmaceutics and Biopharmaceutics, 2021, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2021.10.013.
On the 24th of November is World Diabetes Day. Have a look at our background page:


