Design, Development, and Characterization of Amorphous Rosuvastatin Calcium Tablets
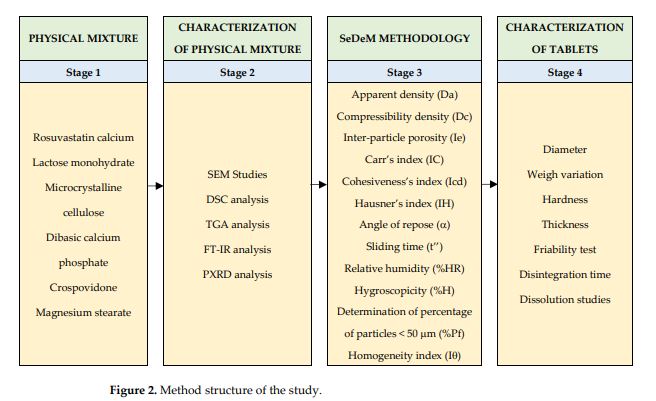
This work proposes a methodology for the design, development, optimisation, and evaluation of amorphous rosuvastatin calcium tablets (BCS class II drug). The main goal was to ensure rapid disintegration and high dissolution rate of the active ingredient, thus enhancing its bioavailability. The design started from a careful selection of excipients, which due to their characteristics and proportions within the formulation allowed the use of their properties such as fluidity or granulometric distribution. The formulation was characterised using scanning electron microscopy (SEM), differential scanning calorimetry (DSC), thermogravimetry (TGA), Fourier transform infrared spectroscopy (FT-IR) and powder X-ray diffraction (PXRD) methods. The galenic SeDeM methodology was used to establish the profile of the active ingredient-excipient mixture and guarantee its suitability for producing tablets by the direct compression method. The results demonstrate that the amorphous rosuvastatin calcium tablets formulation developed made it possible to obtain costeffective tablets by direct compression with optimal pharmacotechnical characteristics that showed a remarkable disintegration and dissolution rate. The manufactured tablets complied with the pharmacopoeia guidelines regarding uniformity of weight, tablet hardness, thickness, friability, in vitro disintegration time and dissolution profile.
Download the full research paper as PDF: Design, Development, and Characterization of Amorphous Rosuvastatin Calcium Tablets
2.1. Materials and reagents
Rosuvastatin calcium (Insud Pharma) (Figure 1), lactose monohydrate (Guinama), microcrystalline cellulose (Vivapur 12®, JRS Pharma GmbH& CO.KG), dibasic calcium phosphate (Emcompress®, Fagron), crospovidone (PVPP, Sigma-Aldrich) and magnesium stearate (Guinama).
Rocío González , M Ángeles Peña , Norma Sofía Torres and Guillermo Torrado
(www.preprints.org)
© 2021 by the author(s). Distributed under a Creative Commons CC BY license.

