A Quality by Design (QbD) study in direct compression using the most flowable microcrystalline cellulose CEOLUS™ UF-702
OBJECTIVE
CEOLUSTM UF-702 is the most flowable microcrystalline cellulose (MCC). While UF-702 has higher compactibility than standard grade PH-102, it has the lowest repose angle of known commercial grades of MCC. This is because the UF-702 particle has a round shape and has many pores inside its particle.
The purpose of this QbD study is to demonstrate that UF-702 has higher robustness compare to PH-102 by analyzing the impact of formulation and process variables on tablet’s critical quality attributes (CQAs) in direct compression of poorly compactible and flowing drug.
What is CeolusTM UF-702?
CEOLUSTM UF-702 is an NF/EP/JP compatible MCC.
Powder properties
Tab.1 Powder properties of CEOLUSTM grades:
| Bulk density (g/cm3) | Av.particle diameter (µm) | Repose Angle (o) | |
| UF-711 | 0.22 | 50 | 42 |
| UF-702 | 0.29 | 90 | 34 |
| KG 1000 | 0.12 | 50 | 57 |
| KG 802 | 0.21 | 50 | 49 |
| PH-101 | 0.29 | 50 | 45 |
| PH-102 | 0.30 | 90 | 42 |
Particle morphology
Fig.2 Particle morphology of CEOLUSTM grades:

Fig.3 Relationship between compactibility and flowability of MCC grade:
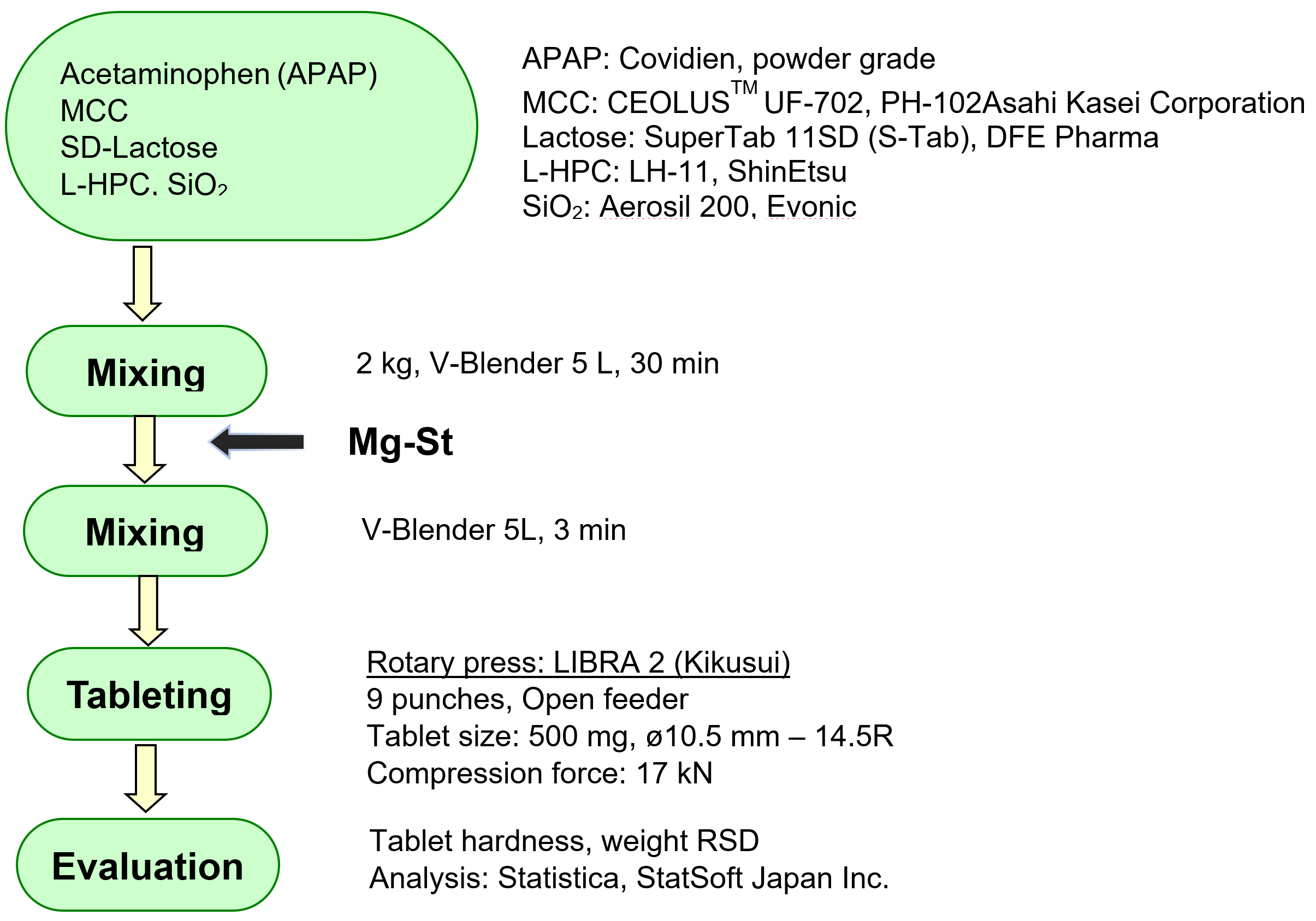
METHOD

Formulation and Process variables
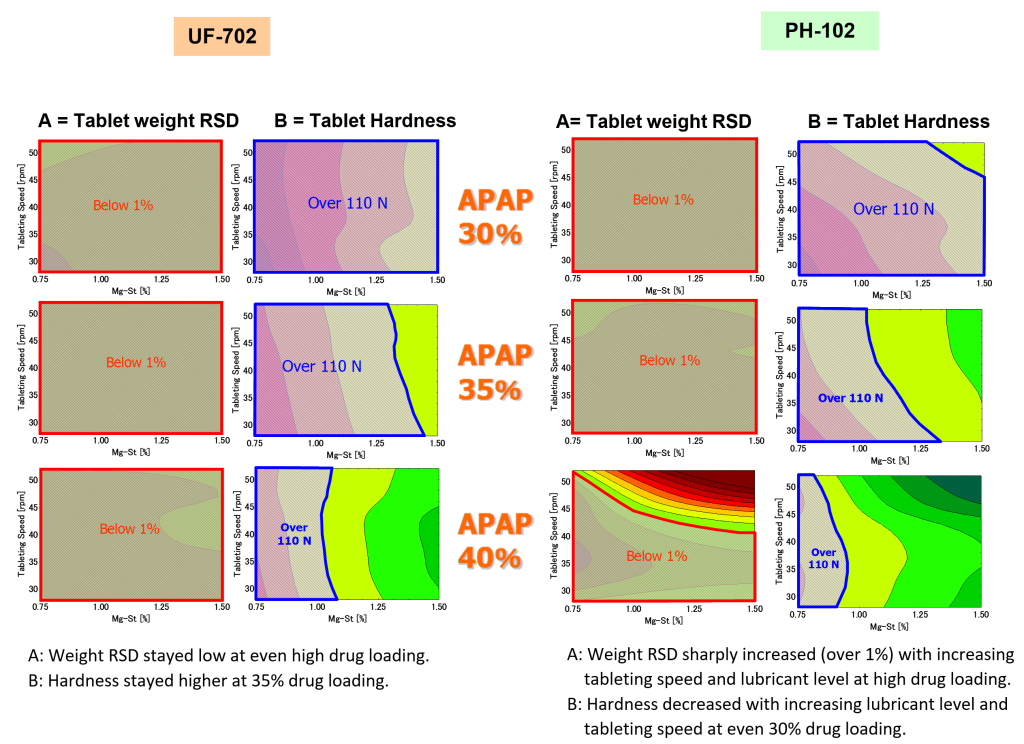
1) Drug Loading, 2) Lubricant level, 3) Tableting speed.
Asahi Kasei compared the robustness between UF-702 and PH-102 by measuring design space, where each hardness was over 110 N and weight RSD was below 1%.
RESULTS
with fixed APAP
UF-702 had wider design space than PH-102.
UF-702 has lower lubricant sensitivity than PH-102.
CONCLUSION
In case of CEOLUSTM UF-702, formulation and process variables had less impact on tablet’s CQAs than PH-102.
This QbD study demonstrated that CEOLUSTM UF-702 formulation has a higher robustness than PH-102 formulation in direct compression of low compactible and flow drug.
REFERENCE
The data of this page is a property of Asahi Kasei Corporation.