Soft sensor for content prediction in an integrated continuous pharmaceutical formulation line based on the residence time distribution of unit operations
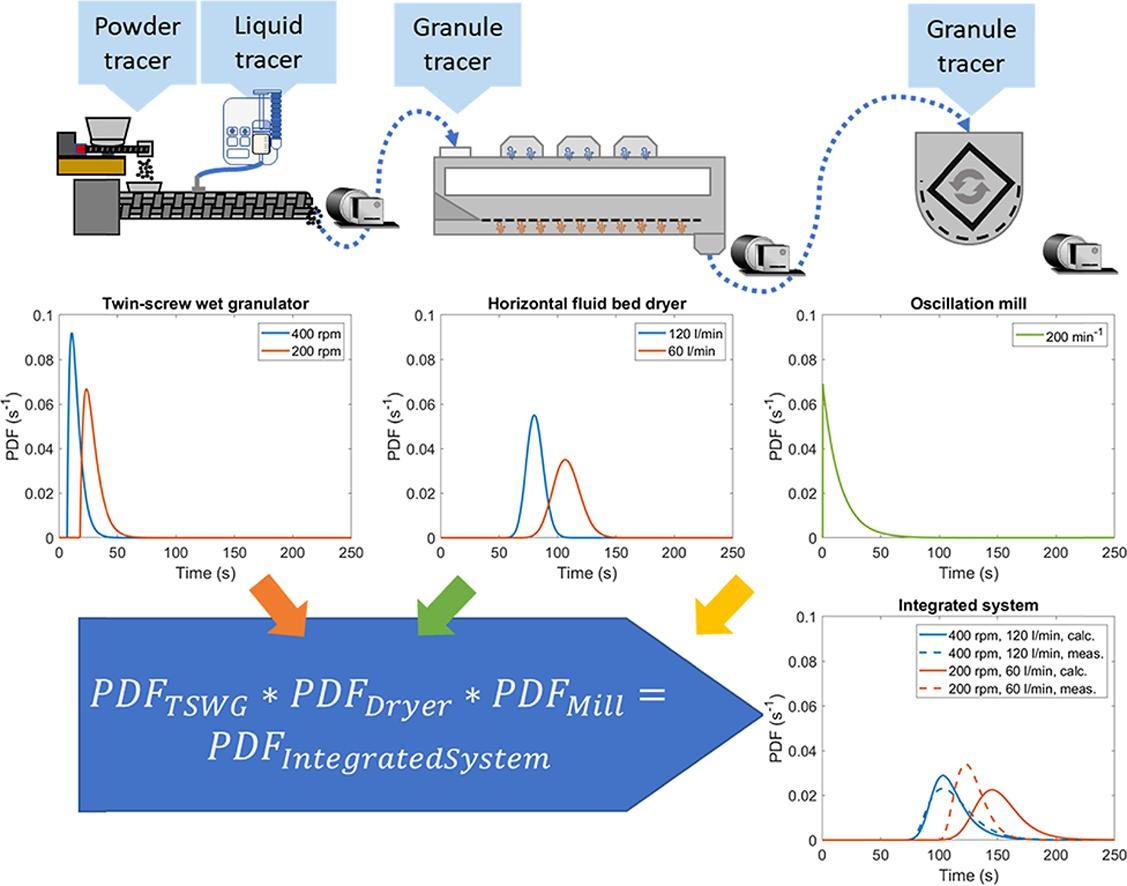
In this study, a concentration predicting soft sensor was achieved based on the Residence Time Distribution (RTD) of an integrated, three-step pharmaceutical formulation line. The RTD was investigated with color-based tracer experiments using image analysis. Twin-screw wet granulation (TSWG) was directly coupled with a horizontal fluid bed dryer and an oscillating mill.
Highlights
- RTD for the two separate input ports of the TSWG is measurable individually.
- CPPs affect the RTD of horizontal fluid bed dryer.
- Convolution based in silico RTD coupling with three-unit operations was validated.
- Soft sensor for ultra-low-dose API content monitoring was comparable to HPLC.
Based on integrated measurement, we proved that it is also possible to couple the unit operations in silico. Three surrogate tracers were produced with a coloring agent to investigate the separated unit operations and the solid and liquid inputs of the TSWG. The soft sensor’s prediction was compared to validating experiments of a 0.05 mg/g (15% of the nominal) concentration change with High-Performance Liquid Chromatography (HPLC) reference measurements of the active ingredient proving the adequacy of the soft sensor (RMSE < 4%).
Download the full article as PDF here Soft sensor for content prediction in an integrated continuous pharmaceutical formulation line based on the residence time distribution of unit operations
or read it here
Materials
Carvedilol (CAR, Sigma-Aldrich, Budapest, Hungary) with a purity of ≥ 98 % and a melting point of 117°C was selected as a model active pharmaceutical ingredient (API). The API was dissolved in 96% ethanol (Sigma-Aldrich, Budapest, Hungary) along with Kollidon® 30 (Povidon, PVPK30, BASF, Ludwigshafen, Germany). Fine α-lactose monohydrate (GranuLac® 230 mesh, Meggle Pharma, Wasserburg, Germany) and potato starch (Roquette Pharma, Lestrem, France) were used as the solid excipients for the granulation.
Martin Gyürkés, Lajos Madarász, Petra Záhonyi, Ákos Köte, Brigitta Nagy, Hajnalka Pataki, Zsombor Kristóf Nagy, András Domokos, Attila Farkas,
Soft sensor for content prediction in an integrated continuous pharmaceutical formulation line based on the residence time distribution of unit operations, International Journal of Pharmaceutics, Volume 624, 2022, 121950, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2022.121950.

