Industry Perspective on the Use and Characterization of Polysorbates for Biopharmaceutical Products Part 2: Survey Report on Control Strategy Preparing for the Future
Abstract
Polysorbate (PS) 20 and 80 are the main surfactants used to stabilize biopharmaceutical products. Industry practices on various aspects of PS based on a confidential survey and following discussions by 16 globally acting major biotechnology companies is presented in two publications. Part 1 summarizes the current practice and use of PS during manufacture in addition to aspects like current understanding of the (in)stability of PS, the routine QC testing and control of PS, and selected regulatory aspects of PS.1 The current part 2 of the survey focusses on understanding, monitoring, prediction, and mitigation of PS degradation pathways in order to propose an effective control strategy. The results of the survey and extensive cross-company discussions are put into relation with currently available scientific literature.
Introduction
The body of research and publications related to polysorbate (PS) in the past few decades have significantly improved our understanding of PS structure, its degradation pathway(s), and consequences of PS degradation on critical quality attributes of biopharmaceuticals. This has contributed to our current state and common practice for handling and control of polysorbates within the biopharmaceutical industry, as presented extensively in Part 1 of this survey report.1
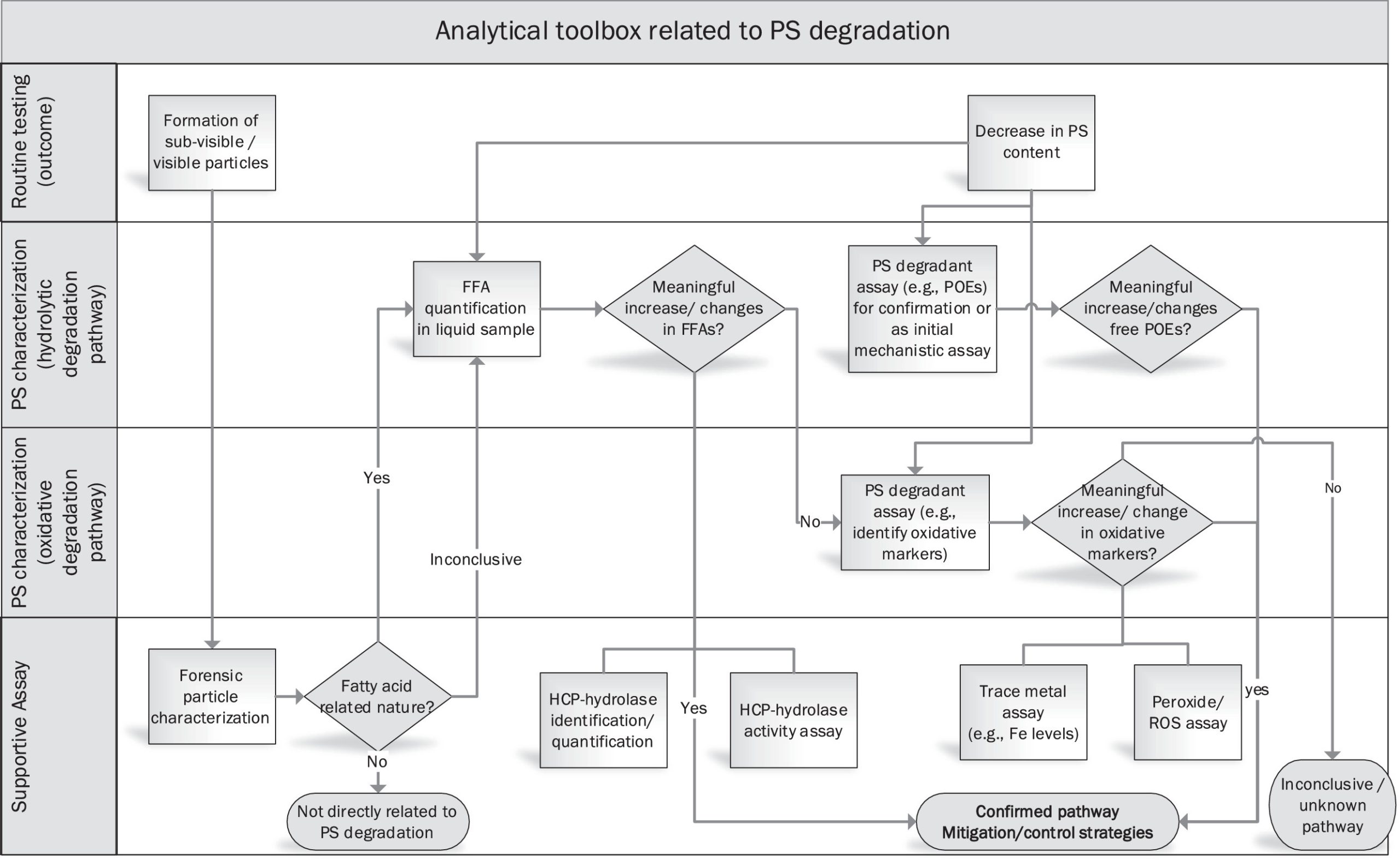
As discussed in a recent USP Polysorbate stimuli article2 and at the CASSS CMC Strategy Forum 2020,3 there are still numerous challenges to overcome in the biopharmaceutical industry to achieve a thorough understanding of PS composition, structure-function relationship and degradation mechanisms to achieve a robust control strategy. These challenges can be summarized into 4 main categories. 1.) PS structure diversity; as discussed in detail in our Part 1 report, commercially available polysorbates are chemically diverse mixtures – the composition and distribution of PS subspecies and impurities vary between manufacturers, grades, as well as lots. This makes PS product sourcing, sample handling, and quality control testing important aspects of the PS control strategy as an excipient in biopharmaceutical formulations. 2.) PS analytical characterization; multiple analytical challenges arise from the fact that PS lacks strong chromophores for commonly used detection by UV-Vis or fluorescence. The universal detectors such as charged aerosol detector (CAD) or evaporative light scattering detector (ELSD) used for PS quantification have shortcomings, e.g., lack of specificity for individual PS components and non-linear response. In addition, no single method can provide a holistic view of PS composition due to its chemically diverse nature. 3.) PS degradation; PS as a protein stabilizer can also degrade via two major pathways. As discussed in Part 1,1 PS degradation is a complex issue, and a clear and simple root cause may not always be identified. In addition, an increasing number of publications indicate that host cell derived hydrolytic enzymes are causing PS degradation in biopharmaceutical solutions.4, 5, 6, 7, 8 Enzymatic cleavage of ester-bonds in PS can increase the level of free fatty acids (FFAs) in the drug product, which may eventually agglomerate and form FFA particles. Hydrolytic enzymes often represent only a small fraction of the total amount of host cell protein (HCP) contaminants typically present in biopharmaceutical drug products which makes their identification and control a challenging task. 4.) PS protein stabilizing mechanism; we do not yet have complete knowledge and understanding on structure-function relation of this excipient. For example, it is not clear if/how the heterogeneous nature of PS contributes to its properties of being a highly effective surfactant and protein stabilizer. In order to proactively mitigate negative effects of PS degradation and its potential impact on product quality, an end-to-end control strategy encompassing PS product sourcing and handling, manufacturing process control, and drug substance (DS) and/or drug product (DP) release and stability monitoring is needed. Current state and common practices for handling of PS as an outcome of an industry survey were summarized in Part 1.1
Part 2 builds on Part 1 survey report and focuses on: 1.) current state of mechanistic understanding of polysorbate degradation pathways; 2.) analytical methods for comprehensive polysorbate characterization; 3.) predictive PS degradation models; and 4.) strategies to control and mitigate PS degradation. It is expected that the control and mitigation strategies will continue to evolve with increasing knowledge of PS degradation, predictive modeling, new and improved analytical methods, as well as the availability of PS alternatives.
Download the full review as PDF here Industry Perspective on the Use and Characterization of Polysorbates for Biopharmaceutical Products Part 2: Survey Report on Control Strategy Preparing for the Future
or read it here
Klaus Wuchner, Linda Yi, Cyrille Chery, Felix Nikels, Friederike Junge, George Crotts, Gianluca Rinaldi, Jason A. Starkey, Karoline Bechtold-Peters, Melissa Shuman, Michael Leiss, Michael Jahn, Patrick Garidel, Rien de Ruiter, Sarah M. Richer, Shawn Cao, Sebastian Peuker, Sylvain Huille, Tingting Wang, Virginie Le Brun, Industry Perspective on the Use and Characterization of Polysorbates for Biopharmaceutical Products Part 2: Survey Report on Control Strategy Preparing for the Future, Journal of Pharmaceutical Sciences, Volume 111, Issue 11, 2022, Pages 2955-2967, ISSN 0022-3549,
https://doi.org/10.1016/j.xphs.2022.08.021.

