Recent advancements in additive manufacturing techniques employed in the pharmaceutical industry: A bird’s eye view
Abstract
The 3–dimensional printing process (3DP) was patented in the 1980s, but the utilization of this process has expanded substantially over the past decade, to which the pharmaceutical industry is a major contributor. With increasing interest, researchers across the globe are striving for the fabrication of novel pharmaceutical dosage forms, especially tailored ones, which can cater to the specific needs of the patient. These dosage forms intend to cater for on–demand manufacturing, personalized medications, enhanced geometry, size, and dosage, and increased bioavailability of the medicinal active. With the emergence of precision medicine in healthcare, the inclusion of additive manufacturing (AM) technologies is deemed imperative for the fabrication of oral dosage forms and, which opens new horizons for the administration of drug combinations and formulations tailored to individual needs. Although the extensive commercialization and acceptance of the AM techniques may disrupt the current healthcare supply chain, it has the potential to curtail the waste produced by expired and unused medications. This article attempts to outline these additive manufacturing techniques of great interest in the pharmaceutical industry while underscoring the current innovative trends pertaining to the 3D printing of pharmaceutical dosage forms, as well as their advantages, limitations, and prospects in the field of research and development. The article also showcases the viability of various 3D printing techniques by citing numerous papers in which said techniques have been successfully exploited to deliver unique pharmaceutical formulations.
Introduction
At the outset, the pharmaceutical industry is growing by leaps and bounds, and recent innovations have certainly facilitated the development of novel dosage forms for targeted therapy. Nonetheless, manufacturing these pharmaceutical dosage forms on an industrial level is still limited and continues to rely on traditional drug delivery systems, primarily in modified tablets. The inception of 3−dimensional printing (3DP) technology has pushed the boundaries of the research and development of novel dosage forms, especially in personalized and modified tablets [1].
Although traditional dosage form manufacturing is meant for mass production, it has certain shortcomings, namely high capital expenditure for acquiring the major equipment, the requirement of a large operating space, a well−trained and adept workforce, and lack of flexibility in dose adjustment. Additionally, it lacks the flexibility in bringing tailored medicine to reality, owing to the lack of flexibility and multifarious process [2]. In the cases of solid unit dosage forms, dose modifications are achieved by dispensing several low−dose tablets that would produce a greater dose or via breaking or dividing high−dose tablets [2]. In the United States, approximately 3000 compounding pharmacies fill over 30 million prescriptions a year, in an effort to personalize the medications for individual patients [2,3]. The splitting of tablets is mainly achieved by means of tablet splitters, hands, or knives, resulting in varying doses, due to dissimilar weight distribution post splitting [4−6]. Tablet splitting could also have a profound effect on the drug release profiles, especially in the extended or controlled release formulations [7,8]. Furthermore, its fractionation has a direct effect on the integrity of the tablet coating, thereby promoting premature drug release [2].
Conventional treatment of patients with a standard dose of a drug can sometimes lead to trial−and−error, suboptimal treatment, and prolong time to establish the optimal dose. This not only leads to a higher treatment cost to the patients but also substantially increases the morbidity and mortality of the patients [9]. This problem can be settled by the individualization of the treatment regimen, which significantly reduces the risk of Adverse Drug Reactions (ADRs) [10]. The Personalized Medication (PM) can potentially tailor the treatment therapy to deliver the best response with the highest margin of safety, to ensure better care of the patients, with lower incurred costs [11]. Although the extemporaneous compounding of personalized medicine is important, compounded preparations pose a multitude of drawbacks, including lack of quality control, variable drug absorption across biological membranes, and unknown stability Parameters [12].
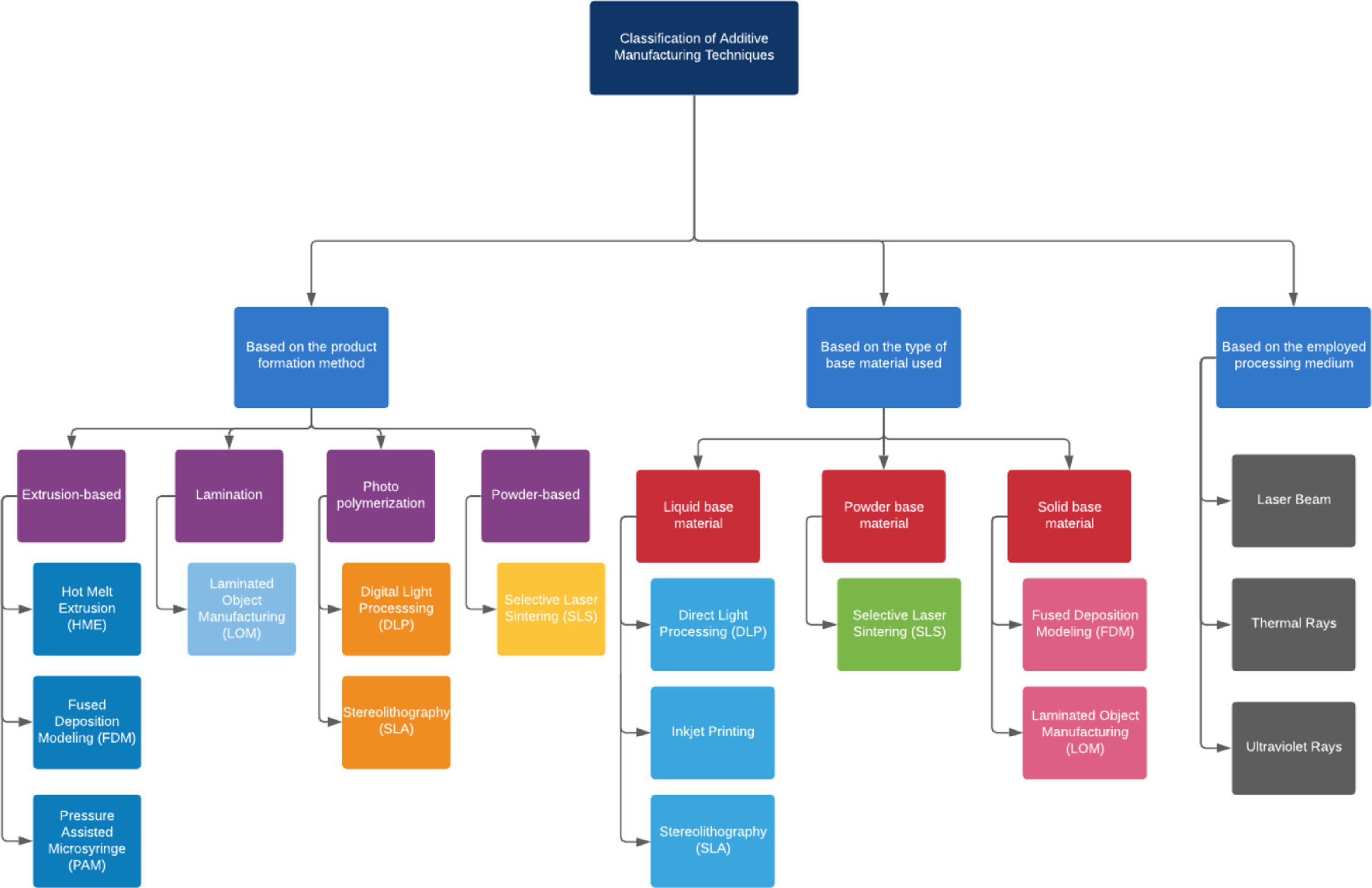
3D Printing (3DP) or Additive Manufacturing (AM) has become one of the most innovative technologies in the pharmaceutical sector, with the last decade witnessing a significant expansion in the manufacture of drug delivery models. 3DP or AM technologies include a plethora of processes in which a solid object is created in a layer−by−layer process [13]. The AM facilitates the creation of Pharmaceutical dosage forms by means of computer−aided designing (CAD), which in turn generates a computer−designed model that fabricates the desired product using layer upon layer feed deposition. Additionally, AM also provides an innovative platform to overcome the limitations attributed to the conventional ’one−size−fits−all’ concept. The most commonly used 3DP technologies employed in pharmaceutical companies include electron beam melting (EBM), extrusion−based 3D printing, inkjet printing, multijet fusion (MJF), powder bed deposition, selective laser melting, selective laser sintering (SLS), and direct metal laser sintering (SLM/DMLS), and tereolithography (SLA). Owing to the multitude of desirable features like flexibility with the design and polymers used, wide availability, and low operational charges, extrusion−based 3DP has portrayed immense potential and interest among researchers [3,14,15].
Extrusion−based 3DP technologies are classified as direct powder extrusion (DPE), pressure −assisted microsyringe (PAM) and fused deposition modeling (FDM) technologies, based on variations in process parameters, as well as the nature and type of polymers used [16]. Direct powder Extrusion (DPE) technique involves the use of a single−screw direct powder extruder 3D printer which was fabricated for printing with polylactic acid (PLA) or acrylonitrile butadiene styrene (ABS). In this technique, a small spatula is employed to add the mixture into the hopper of the printer and to push the material inside the single−screw extruder. The extruder is placed vertically that facilitates the flow of powder into the screw and also decreases the presence of air bubbles. Furthermore, pressure−assisted microsyringe is used to produce hybrid film structures while circumventing the problem of blending immiscible polymers. This technique is useful in determining the chemical structure, morphology, mechanical properties and disintegration [17].
Lastly, Fused deposition modeling (FDM) is an additive or anabolic process that involves building components by addition of material [18]. The next part of the review will briefly underscore the various 3DP−based technologies, that precede the development and manufacture of personalized dosage forms.
Table 2
Recent advancements of the AM Techniques in formulating various pharmaceutical dosage forms.
| Techniques | AM Study | API | Excipients | Remarks | Reference |
|---|---|---|---|---|---|
| Stereolithography (SLA) | Multilayer 3D printed oral dosage form (polyprintlet) | Irbesartan, atenolol, hydrochlorothiazide, and amlodipine | Polyethylene glycol diacrylate (PEGDA), Diphenyl (2, 4, 6−trimethyl−benzoyl) phosphine oxide (TPO), Polyethylene glycol (PEG 300), Acetonitrile | A multilayered antihypertensive polypill was successfully fabricated to deliver low−dose combination therapy. | [209] |
| Tablets loaded with drugs with modified drug release profiles | 4−aminosalicylic acid (4 −ASA) and Paracetamol (acetaminophen) | Diphenyl (2,4,6−trimethyl benzoyl) phosphine oxide (DPPO), Poly (ethylene glycol) diacrylate (PEGDA), Poly (ethylene glycol) (PEG 300) | Varying the percentage of cross−linkable polymers in the tablets modulates the drug dissolution profiles. Higher ratios of PEGDA reduce the dissolution rate, while a higher concentration of PEG 300 promotes drug release. | [210] | |
| Ascorbic acid−loaded solid dosage Hydrogels | Ascorbic acid, Riboflavin | Poly (ethylene glycol) dime thacrylate (PEGDMA), triethanolamine, phosphate buffer (pH 6.8), phosphoric acid, methanol, hydrochloric acid | This work showed the ability of SLA 3D printing to successfully release a bioactive molecule from a single formulation in a controlled manner. | [211] | |
| Ibuprofen−loaded cross −linked polyethylene glycol diacrylate (PEGDA) hydrogels | Ibuprofen | Polyethylene glycol diacrylate (PEGDA), polyethylene glycol (PEG 300), riboflavin, triethanolamine (TEA), diphenyl (2,4,6− trimethyl benzoyl) phosphine oxide (DPPO) | SLA is a suitable technique that can be used to prepare pharmaceutical hydrogels. | [212] | |
| 3DP of a multilayer polypill containing 6 drugs | Paracetamol, Caffeine, Naproxen, Chloramphenicol, Prednisolone, Aspirin | Polyethylene glycol diacrylate (PEGda) and diphenyl (2,4,6−trimethyl benzoyl) phosphine oxide (TPO) | Cylindrical and ring−shaped polypills with and without a soluble filler were made that showed acceptable physicochemical characteristics and various combinations of the physicochemical properties. Drug Release Profiles | [50] | |
| Riboflavin and ibuprofen hydrogels | Riboflavin, Ibuprofen | Polyethylene glycol diacrylate, polyethylene glycol (PEG300) | Prepared drugs have a controlled release capacity. | [212] | |
| Inkjet Printing | Controlled release acetaminophen tablets | Acetaminophen | Hydroxypropyl methylcellulose E100, Ethyl cellulose, Polyvinylpyrrolidone K30 (PVP K30), colloidal silicon dioxide | The release efficiency of poorly water−soluble drugs was enhanced by combining them with feuhydrophilic polymers. | [213] |
| Controlled release rates of two types of Chlorpheniramine maleate tablets. One with Eudragit E−100 as polymer and another with Eudragit RLPO as polymer. | Chlorpheniramine maleate | Eudragit E−100, ethanol, Eudragit RLPO, | The release rate varied for both the tablets and was based on the quantity of polymer used | [214] | |
| Controlled release of Chlorpheniramine maleate, diclofenac tablets | Chlorpheniramine maleate, diclofenac | Eudragit E−100, ethanol, Eudragit RLPO, Avicel PH301, DCL11 spray −dried lactose, Kollidon K −25, methanol | Prepared tablets contained a quick dissolve region to break the tablet into controlled regions and the release rate was measured. | [215] | |
| The rapid release rate of levetiracetam tablets | Levetiracetam | Colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, polyethylene glycol 3350, polyethylene glycol 6000, polyvinyl alcohol, talc, titanium dioxide. | Prepared tablets disperse in less than 15 s in the mouth and exhibit high release rates. | [216] | |
| Zero−order controlled release Pseudoephedrine HCl formulation | Pseudoephedrine HCI | Kollidon SR, Hydroxypropylmethylcellulose (HPMC) | Zero−order controlled release pseudoephedrine HCl formulations were prepared, and the drug release rate was altered by modulating the number of polymers used. | [217] | |
| Fused Deposition Modeling | Controlled release of progesterone by vaginal rings of different shapes. | Progesterone. | Polyethylene glycol (PEG), polycaprolactone (PCL), and polylactic acid (PLA). | A seven−day controlled release was observed | [42] |
| Controlled release Acetaminophen tabs | Acetaminophen | Polylactic acid (PLA), Cellulose (EC/HPC/HPMC)/Eudragit L100. | The printed tabs had a consistent appearance with the extended drug release property. | [218] | |
| Controlled Indomethacin Release Tabs | Indomethacin | Ethylene−vinyl acetate, Sodium chloride, Absolute ethanol, Purified water | The burst release of the drug was followed by a slow diffusion in the matrix | [219] | |
| Controlled Deflazacort Release Tabs | Deflazacort | Poly(e−caprolactone) (PCL), Eudragit RL100 (ERL), mannitol (Channelling agent) | The prepared tablets had a partially hollow core (50%), a high drug loading (0.27% w/w) & faster drug release | [220] | |
| Controlled Paracetamol release tabs | Paracetamol | Hypromellose acetate succinate, Methylparaben, magnesium stearate | Prepared tablets had 20% infill capacity and different drug release rates were observed in different phases. | [221] | |
| Controlled Theophylline release tabs | Theophylline | Methacrylic polymers (Eudragit RL, RS, and E)/HPC, Hydroxypropyl cellulose, Triethyl 110 citrate (TEC), Triacetin | The thermal analysis reported crystalline structure of theophylline and drug release rate was determined | [222] | |
| Thermal non−degradable and controlled release potent fluorescein tabs | Fluorescein | Polyvinyl alcohol (PVA), Absolute ethanol, | The prepared tablets were mechanically strong and no thermal degradation was reported. The controlled release profile was also reported. | [15] | |
| Controlled release Budesonide tablets | Budesonide | Polyvinyl alcohol, Eudragit L100, Cortiment, Entocort1 CR | The drug began its release in the middle of the small intestine and continued until the distal intestine and colon. Therefore, it has a controlled release ability. | [223] | |
| Controlled release Prednisolone tablets | Prednisolone | Polyvinyl alcohol, glycerol, acetonitrile, and methanol | The precision control of the drug ranged between 88.7% and 107%. Prednisone is present in amorphous form and the release could increase up to 24 h with the use of 3d printing. | [222] | |
| Laminated Object Modelling | Modified release, 4 ASA and 5 ASA tablets | 5−aminosalicylic acid (5 −ASA, mesalazine), 4 −aminosalicylic acid (4 −ASA) | Polyvinyl alcohol (PVA) | 4 ASA tablets were degraded about 50% during the process, while on the other hand 5 ASA tablets were not degraded and were mechanically stable. | [224] |
| Hot Melt Extrusion | Pelvis model manufacturing | --- | Polyethylene tubercle | Pelvis model was prepared with equal proportion (1:1) to the patient’s pelvis | [225] |
| Glass solution formation of poorly water−soluble drugs | Indomethacin, nifedipine, tolbutamide. | Polyvinylpyrrolidone (PVP), Vinyl acetate (VA) | A crystalline structure was detected, which indicated an incomplete melting point of the drug. | [226] | |
| Preparation of Nifedipinevtablets by kneading the paddle element. | Nifedipine | Hydroxypropylmethylcellulose phthalate (HPMCP) | Kneading paddle elements of twin−screw extruders play a significant role in the transformation of the crystalline form to the amorphous form. | [227] | |
| Stability of Polyethylene oxide (PEO)in Chlorpheniramine Maleate tablets. | Chlorpheniramine Maleate | Polyethylene oxide (PEO). | The prepared tablets were sensitive to both temperature and screw speed. | [124] | |
| A starch−based formulation for preparation of Theophylline tablets | Theophylline | Starches and sugar alcohols | Sustained drug release was observed and no significant effect on water content and porosity was reported. | [228] | |
| Stability determination of 17b−estradiol hemihydrate Tablets prepared by extrusion. | 17b−estradiol hemihydrate | Estradiol, Polyvinylpyrrolidone (PVP), Sucroester WE15, magnesium stearate | The study was based on the preparation of 17b−estradiol hemihydrate tablets that do not recrystallize after extrusion as stability could decrease due to the recrystallization process. | [230] | |
| On−demand warfarin release tablets | Warfarin | Eudragit E, triethyl citrate (TEC), acetonitrile, tricalcium phosphate (TCP) | Prepared tablets were dynamic and responses could be set according to patients’ profile | [231] | |
| Non−destructive dose verification paracetamol tablets | Paracetamol | L−HPC, mannitol, magnesium stearate | The prepared drug has non −destructive property and rapid release property | [146] | |
| Controlled release Guaifenesin tablets | Guaifenesin | Hydroxypropyl methylcellulose, Polyacrylic acid, Carbopol NF, hydroxypropyl methylcellulose (HPMC) | The release rate of all formulations had an n−value between 0.27 and 0.44 thereby indicating the Fickian diffusion drug release pattern. | [167] | |
| Controlled release Acetaminophen tablets | Acetaminophen | Polyethylene glycol, polyvinyl acetate, and polyvinyl caprolactam, hydroxypropyl methylcellulose | The prepared drug showed a steady release rate (Zero order) | [232] | |
| Dapivirine releasing vaginal rings | Dapivirine | Thermoplastic polyurethanes PY−PT87AE (T87) and PY−PT60DE (T60), e isopropyl alcohol (IPA), acetonitrile (ACN), methanol, and acetone | Drug loading in the vaginal rings was convenient and the dose could be altered depending on the patients | [233] | |
| Selective Laser Sintering | Oral disintegrated Ondansetron tablets | Ondansetron, cyclodextrin | Mannitol, Kollidon VA64, Candurin, Gold Sheen | Prepared tablets were formulated in cyclodextrin complexes and high conc. mannitol and possessed fast disintegration (15 s) and 90% of the drug was disintegrated in about 5 mins. | [151] |
| pH dependent, Sustained release Paracetamol tablets | Paracetamol | Kollicoat IR, polyvinyl alcohol, polyethylene glycol copolymer, and Eudragit L100−55 | The prepared drug was pH. dependent and with a complete release of approximately 12 h. | [147] | |
| Diclofenac sodium solid dosage 3d printed drug | Diclofenac sodium | Kollidon VA64, Lactose monohydrate, Candurin NXT Ruby Red | Prepared tablets possessed good mechanical stability, a high rate of integration and dissolution rates. No chemical reactions between components and crystalline structure were reported | [1] | |
| The drug release pattern of Progesterone tabs formulated with PCL | Progesterone | Polycaprolactone (PCL) | The drug release pattern was linear and possessed zero −order kinetics. | [234] | |
| Miniprintlet preparation consisting of Paracetamol & Ibuprofen | Paracetamol, Ibuprofen | Ethyl cellulose, Kollicoat Instant release (IR) | The prepared drug was very flexible and the drug content and release properties could be modified. | [145] | |
| Fabrication of polymeric drug delivery devices (DDD) | Methylene blue | Polyamide (PA), phosphate buffer solution (PBS) | The devices could retard and release the drug in a sustained manner. | [235] | |
| Pressure−Assisted Microsyringe | Immediate release Levetiracetam tablets | Levetiracetam | Polyvinyl alcohol, polyethylene glycol, polyvinylpyrrolidone−vinyl acetate | The prepared tablet had an API in the amorphous form which exhibited stability for 3 months. | [162] |
| Pediatric dose Levetiracetam tablets | Levetiracetam | Polyvinyl alcohol−polyethylene glycol, Di−sodium hydrogen phosphate dihydrate, potassium dihydrogen orthophosphate | The prepared dosage form disintegrated quickly, facilitating use as a pediatric dose. Splitting the tablet into multiple layers led to less API concentration for pediatric patients. | [201] | |
| Sustained release of levetiracetam tablets | Levetiracetam | Polyvinyl acetate/polyvinyl pyrrolidone (PVAc−PVP), hydroxypropylmethylcellulose (HPMC), silicon dioxide (SiO2) | The release rate could be controlled by the amount of polymer used and the drugs exhibited great mechanical stability. | [178] | |
| Direct Powder Extrusion | Fabrication of amorphous solid itraconazole dispersions | Itraconazole | HPC−UL (MW 20,000), HPC −SSL (MW 40,000), HPC −SL (MW 1,00,000) and HPC−L (MW 1,40,000). | Dispersions fabricated using HPC−UL (ultra−low MW) showed drug release faster than those of the other HPC grades. | [191] |
| Preparation of 3DP tablets of amorphous solid dispersions for pediatric use | Praziquantel | Kollidon (KOL), Kolliphor SLS Fine, Acetonitrile. | Printlets showed improved performance in performance studies, along with acceptable taste thresholds. | [236] | |
| DPE of paracetamol−loaded mixtures via low thermal processing | Paracetamol (acetaminophen 98%) | Potato starch, Hydroxypropyl cellulose, Guar gum, Hydrochloric acid, Acetonitrile | The applicability of this mix for customized drug development at low temperatures and without the requirement for specific equipment was demonstrated. | [194] |
Download the full article as PDF here Recent advancements in additive manufacturing techniques employed in the pharmaceutical industry: A bird’s eye view
or read it here
Ryan Varghese, Sahil Salvi, Purab Sood, Jainam Karsiya, Dileep Kumar, Recent advancements in additive manufacturing techniques employed in the pharmaceutical industry: A bird’s eye view, Annals of 3D Printed Medicine, Volume 8, 2022, 100081, ISSN 2666-9641,
https://doi.org/10.1016/j.stlm.2022.100081.

