Trends in oral small-molecule drug discovery and product development based on product launches before and after the Rule of Five
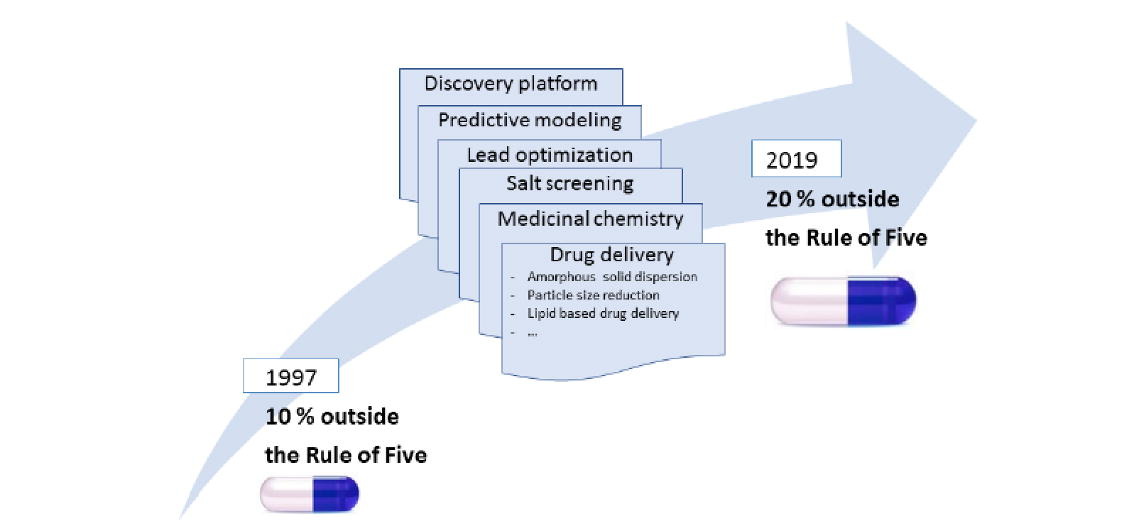
In 1997, the ‘Rule of Five’ (Ro5) suggested physicochemical limitations for orally administered drugs, based on the analysis of chemical libraries from the early 1990s. In this review, we report on the trends in oral drug product development by analyzing products launched between 1994 and 1997 and between 2013 and 2019. Our analysis confirmed that most new oral drugs are within the Ro5 descriptors; however, the number of new drug products of drugs with molecular weight (MW) and calculated partition coefficient (clogP) beyond the Ro5 has slightly increased. Analysis revealed that there is no single scientific or technological reason for this trend, but that it likely results from incremental advances are being made in molecular biology, target diversity, drug design, medicinal chemistry, predictive modeling, drug metabolism and pharmacokinetics, and drug delivery.
Introduction
Twenty-five years ago, the poor water solubility of small molecules referred to as new chemical entities (NCEs) as a result of the advent of combinatorial chemistry and high-throughput screening (HTS) procedures was recognized as a problem in drug development. The physicochemical descriptors were revealed by searching and analyzing chemical libraries of pharmaceutical companies. As a result, Lipinski et al. proposed the Ro5, which is based on limit values for MW ≤500 Da, clogP ≤5, hydrogen bond acceptors (HBAs) ≤10, and hydrogen bond donors (HBDs) ≤5. If not more than one parameter exceeds the Ro5 parameter, the compound can still be considered generally within the Ro5.1 The Ro5 not only brought the poor solubility of NCEs into scientific focus, but also sparked considerable debate among scientists regarding the proposed Ro5 and its valid limits. Regardless of the different scientific opinions and subsequently proposed rules, the problem of poor water solubility of NCEs was evident, and required appropriate responses from pharmaceutical scientific community. Here, we review the trends in oral drug product development according to the Ro5 to provide insights into the scientific advances as well as approaches the pharmaceutical companies have taken, as far as are publicly known, to solve the problem of poor water solubility and, hence, the bioavailability of NCE throughout the drug development process.
Download the research paper as PDF: Trends in oral small-molecule drug discovery and product development
or continue here with Trends in oral small-molecule drug discovery and product development
Sven Stegemann, Chris Moreton, Sami Svanbäck, Karl Box, Geneviève Motte, Amrit Paudel,
Trends in oral small-molecule drug discovery and product development based on product launches before and after the Rule of Five,
Drug Discovery Today, 2022, 103344, ISSN 1359-6446,
https://doi.org/10.1016/j.drudis.2022.103344.

