Alternative Excipients for Protein Stabilization in Protein Therapeutics: Overcoming the Limitations of Polysorbates
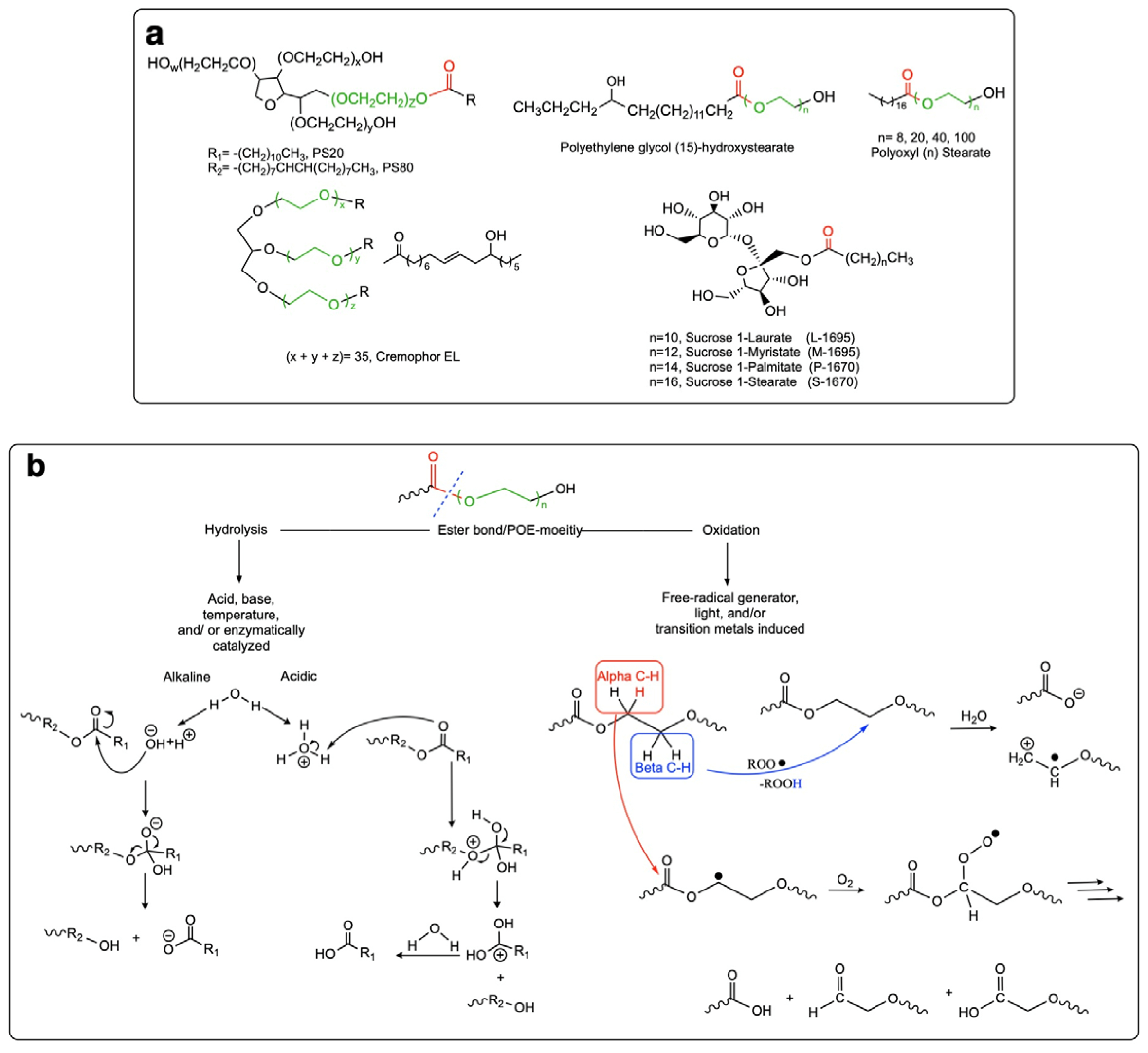
Given their safety and efficiency in protecting protein integrity, polysorbates (PSs) have been the most widely used excipients for the stabilization of protein therapeutics for years. In recent decades, however, there have been numerous reports about visible or sub-visible particles in PS-containing biotherapeutic products, which is a major quality concern for parenteral drugs. Alternative excipients that are safe for parenteral administration, efficient in protecting different protein drugs against various stress conditions, effective in protein stabilization in high-concentrated liquid formulations, stable under the storage conditions for the duration of the product’s shelf-life, and compatible with other formulation components and the primary packaging are highly sought after.
The aim of this paper is to review potential alternative excipients from different families, including surfactants, carbohydrate- and amino acid-based excipients, synthetic amphiphilic polymers, and ionic liquids that enable protein stabilization. For each category, important characteristics such as the ability to stabilize proteins against thermal and mechanical stresses, current knowledge related to the safety profile for parenteral administration, potential interactions with other formulation components, and primary packaging are debated. Based on the provided information and the detailed discussion thereof, this paper may pave the way for the identification or development of efficient excipients for biotherapeutic protein stabilization.
Extract from Table 2. Summary of the available information on the potential alternatives to PSs for the stabilization of protein biotherapeutic formulations.
| Subcategory | Excipients | Protein Stabilization Efficiency | Physicochemical Stability | Regulatory Status and Safety | Comparative Studies to PS |
|---|---|---|---|---|---|
| Surfactants: | Polyethylene glycol (PEG) stearates and PEG fatty esters | polyoxyl 15 hydroxy stearate and polyoxyl 35 castor oil (Kolliphor® HS 15 and Kolliphor® EL): protect amylase and bovine serum albumin (BSA) from chemical and mechanical stresses [204]. As in the case of other surfactants, protection of the protein integrity through direct interaction therewith as well as competitive interface adsorption is expected. | are susceptible to chemical and enzymatic cleavage of ester bond and oxidation of POE [203] | - PEG 2 stearate: FDA-approved for topical application - PEG 8 and 100 stearates: FDA-approved for oral and topical application - PEG 40 stearate: FDA-approved for ophthalmic, oral, and topical applications - polyoxyl 15 hydroxy stearate and polyoxyl 35 castor oil: FDA approval for oral, parenteral, and ophthalmic application - potential PEG-mediated immunogenicity and hypersensitivity | - polyoxyl 15 hydroxy stearate: superior toxicity profile to PS80 [209] - polyoxyl 15 hydroxy stearate and polyoxyl 35 castor oil: comparable efficiency in protecting BSA against mechanical stress for 7 days [204] - polyoxyl 35 castor oil: superior efficiency to stabilize amylase in the presence of H2O2 over a period of two months [204] |
| Block polyethylene-propylene glycols (Poloxamers®) | protect the protein through direct interaction as well as competitive interface adsorption | are susceptible to thermal oxidation of PPO block in solid and liquid states | - P188: FDA-approved for intravenous formulations, part of commercial biotherapeutic formulations such as Gazyva®, Orencia®, Norditropin®, and Hemlibra® - potential PEG-mediated immunogenicity and hypersensitivity | P188: Comparable efficiency with PSs in stabilizing mAb formulations, production of PDMS during long-term storage due to interactions with the silicone oil in the stopper in case of P188 [241], increase of glide force in prefilled syringes by PS, but not P188 | |
| Polyoxyethylene fatty ethers (Brij®) | protect the protein through direct interaction as well as competitive interface adsorption [247,248] | might be susceptible to metal- and photo-induced oxidation [78,251] | - FDA-approved for topical administration - Brij® 58: no side effects when administrated in a concentration of 20 mg/mL in animal toxicological studies [249] | - Brij® 92: comparable efficiency to PSs in stabilizing rhGH [247] - Brij® 35: performed inferior to PSs in improving the mechanical and thermal stability of mAb, but superior in protecting the protein against photo-oxidation [250] - Brij® 58: superior inherent stability of the surfactant in protein formulations when compared to PS20 and PS80 [249] |
|
| Carbohydrates: | Cyclodextrins | protect protein either through direct interaction (in case of certain proteins with substantial solvent exposure to hydrophobic amino-acid residues [311]) or competitive interfacial adsorption (in case of CD with higher surface-active properties [307]), dominant mechanism also depends on protein concentration | are potentially susceptible to enzymatic degradation | - 2-hydroxypropyl-γ-cyclodextrin; FDA-approved for ophthalmic and topical administration - α-cyclodextrin: FDA-approved for intracavitary administration - β-cyclodextrin and sulfobutylether-β-cyclodextrin: FDA-approved for oral, topical, intramuscular, subcutaneous, and intravenous administration (GRAS) | hydroxypropyl-β-cyclodextrin: reduced the interface-induced precipitation of porcine growth hormone through a mechanism similar to PS20 [307], protects PS20 from enzymatic degradation [324] thereby combination of the two might be beneficial for overcoming the drawbacks associated with PS degradation |
| Hydroxypropyl methyl cellulose (HPMC) | efficiently stabilizes mAb formulations such as cetuximab and abatacept [330] | is potentially susceptible to enzymatic degradation | FDA-approved, GRAS excipient for oral, buccal, vaginal, and nasal administration | stabilization of cetuximab comparable with PS80 [330] | |
| Amino acid-based stabilizers: | Amino acids | - certain amino acids promote protein solubilization and refolding mostly by altering protein–solvent interactions, also serve as molecular crowders, stabilizing effect of amino acids is protein dependent - arginine protects protein in solution, and acts as a cryoprotectant [255,256] | are not prone to degradation under formulation and storage conditions | - GRAS - FDA-approved for injectable (intramuscular, intravenous), and oral formulations | Not available |
| Synthetic amphiphilic polymers: | Polyether polyols (PEG) and Polypropylene glycols (PPG) | - PEGs: increase protein stability through covalent binding, protection of the free protein from thermal and lyophilization stresses [373,374] - PPGs: protect cetuximab from agitation-induced stress, potentially through direct interaction with the protein [330] | are potentially susceptible to oxidation | - PEGs: FDA-approved for intravenous administration, immunogenicity, and hypersensitivity to formulations containing PEGs and their derivatives have been reported [226] - PPGs: FDA-approved for oral and topical formulations, cross-reactivity of anti-PEG antibodies with PPGs is possible [378] | PPGs: comparable efficiency with PS80 in protecting cetuximab from agitation-induced stress [378] |
Download the full article as PDF here Alternative Excipients for Protein Stabilization in Protein Therapeutics_Overcoming the Limitations of Polysorbates
or read it here
Castañeda Ruiz, A.J.; Shetab Boushehri, M.A.; Phan, T.; Carle, S.; Garidel, P.; Buske, J.; Lamprecht, A. Alternative Excipients for Protein Stabilization in Protein Therapeutics: Overcoming the Limitations of Polysorbates. Pharmaceutics 2022, 14, 2575. https://doi.org/10.3390/pharmaceutics14122575

