Stability of Oral Liquid Dosage Forms in Pediatric Cardiology: A Prerequisite for Patient’s Safety—A Narrative Review
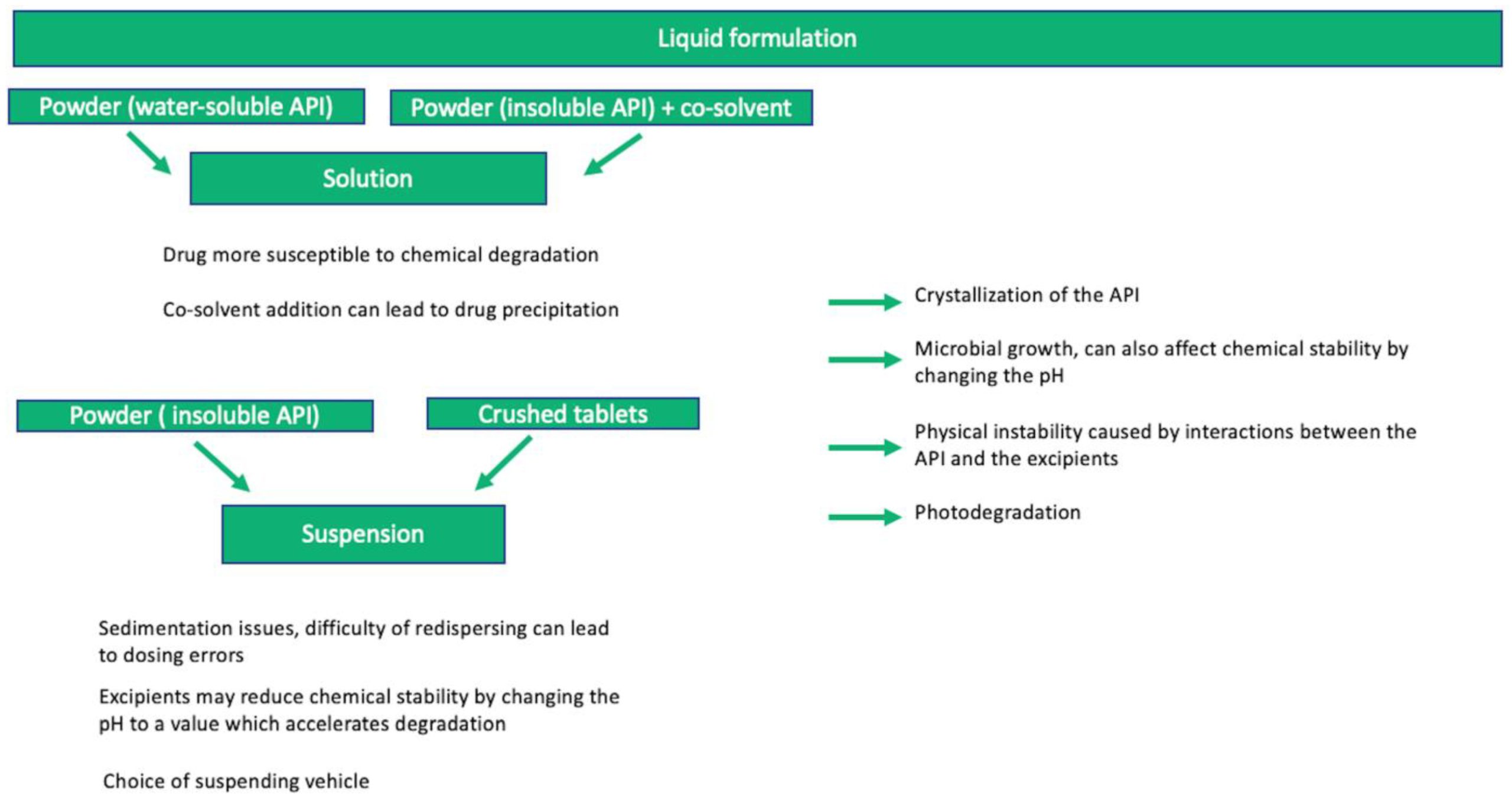
The development of safe and effective pediatric formulations is essential, especially in therapeutic areas such as pediatric cardiology, where the treatment requires multiple dosing or outpatient care. Although liquid oral dosage forms are considered the formulation of choice given the dose flexibility and acceptability, the compounding practices are not endorsed by the health authorities, and achieving stability can be problematic. The purpose of this study is to provide a comprehensive overview of the stability of liquid oral dosage forms used in pediatric cardiology. An extensive review of the literature has been performed, with a particular focus on cardiovascular pharmacotherapy, by consulting the current studies indexed in PubMed, ScienceDirect, PLoS One, and Google Scholar databases. Regulations and guidelines have been considered against the studies found in the literature. Overall, the stability study is well-designed, and the critical quality attributes (CQAs) have been selected for testing. Several approaches have been identified as innovative in order to optimize stability, but opportunities to improve have been also identified, such as in-use studies and achieving dose standardization. Consequently, the information gathering and the results of the studies can be translated into clinical practice in order to achieve the desired stability of liquid oral dosage forms.
1. Introduction
Download the full review as PDF here: Stability of Oral Liquid Dosage Forms in Pediatric Cardiology: A Prerequisite for Patient’s Safety—A Narrative Review
or read it here
Jîtcă, C.-M.; Jîtcă, G.; Ősz, B.-E.; Pușcaș, A.; Imre, S. Stability of Oral Liquid Dosage Forms in Pediatric Cardiology: A Prerequisite for Patient’s Safety—A Narrative Review. Pharmaceutics 2023, 15, 1306.
https://doi.org/10.3390/pharmaceutics15041306

