The Passive Diffusion Improvement of Vitamin B12 Intestinal Absorption by Gelucire that fit for Commercialized Production
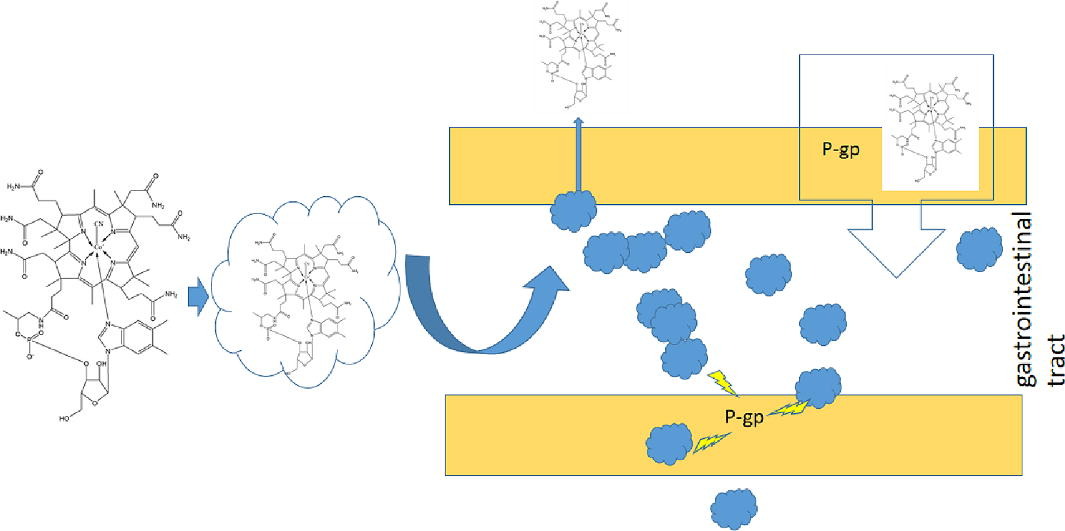
Vitamin B12 (VB12) is a vital micronutrient to maintain the normal state of the hematopoietic system. It must be obtained from the diet since the human body cannot synthesize it. Moreover, the absorption of VB12 needs to be mediated by intrinsic factor on the gastrointestinal (GI) track. The abnormalities in the stomach or lack of such intrinsic factors may result in poor oral absorption of VB12. However, the very advanced formulation strategies were generally very costly and still in the development stage. Thus, the objectives of the present study were to increase the VB12 intestinal absorption by conventional excipients of Gelucire 44/14 (G44/14) or Labrasol, which could be potentially formulated as a cost effect balanced product.
The in vitro Caco-2 cell model was applied for the absorption study. A novel VB12 solid dispersion was subsequently prepared and further characterized by Differential scanning calorimetry, Fourier transform infrared spectroscopy, and Scanning electron microscopy, respectively. The membrane permeability of the VB12 solid dispersion was finally evaluated using ex vivo rat everted gut sac method. The results suggested that G44/14 could significantly enhance the intestinal absorption of VB12 via P-glycoprotein inhibition in vitro (P<0.01). The membrane permeability of VB12could be significantly (P<0.01) improved byG44/14-VB12 solid dispersion at a proportion of carrier: drug ratio of 20:1.The liquidfied solid dispersion was finally directly filled in the hard gelatin capsules. In conclusion, the cheap and simplified process of VB12 complex prepared by G44/14 could potentially increase VB12 intestinal absorption, which may be liable to commercial manufacturing.
2.1. Materials
VB12 standard was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Quinidine and cyclosporin A were obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). Verapamil was from Heowns Biochemical Technology Co., Ltd. (Tianjin, China). Fluorescein sodium standard was purchased from National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, China). Labrasol and G44/14 were generous gifts from Gattefossé (Saint-Priest, France). Kreb’s Ringer Buffer (KRB) was purchased from Yuanye Biochemical Technology Co., Ltd. (Shanghai, China). Caco-2 cells were obtained from Key GENBioTECH Corp., Ltd. (Nanjing, China). Dulbecco’s Modified Eagle’s Medium (DMEM), were obtained from Gibco™ (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Ex Cell Biology, Inc. (Shanghai, China). MTT was from Sangon Biotech Co., Ltd. (Shanghai, China). Hank’s balanced salt solution (HBSS), phosphate buffer saline (PBS) powder, trypsin, penicillin-streptomycin solution (100X) and dimethyl sulfoxide (DMSO, cell culture grade) were obtained from Solarbio Science & Technology Co., Ltd. (Beijing, China). Transwell® Permeable Supports were from Costar® (Corning Inc., USA). The solvents were of the High Performance Liquid Chromatography (HPLC) grade. Other reagents were of the Analytical Reagent (AR) grade.
Download the full study as PDF here: The Passive Diffusion Improvement of Vitamin B12 Intestinal Absorption by Gelucire that fit for Commercialized Production
or read it here
Cheng-Qi Jia, Shu-Yan Wang, Ye Yuan, Yu-Qing Wu, Yan Cai, Jun-Wei Liu, Hai-Qiu Ma, The Passive Diffusion Improvement of Vitamin B12 Intestinal Absorption by Gelucire that fit for Commercialized Production, Saudi Pharmaceutical Journal, 2023, , ISSN 1319-0164,
https://doi.org/10.1016/j.jsps.2023.04.024.

