Risk-based contamination control strategy of manufacturing non-sterile pharmaceutical products
Introduction and aim
Contamination is one of the biggest risks to patients’ health and the quality of pharmaceutical products. According to the new version of Annex 1: Manufacture for sterile medicine products, manufacturers of pharmaceutical products need to develop a Contamination Control Strategy, by implementing the principles of risk analysis, the critical control points are defined and the effectiveness of undertaken controls and measures for management of risks associated with contamination are analyzed. The aim of this paper is to present the advantages and need for implementation of risk assessment as a necessary tool for identifying potential hazards and risks of contamination and manufacturing a product that will meet the quality specification.
Risk tools
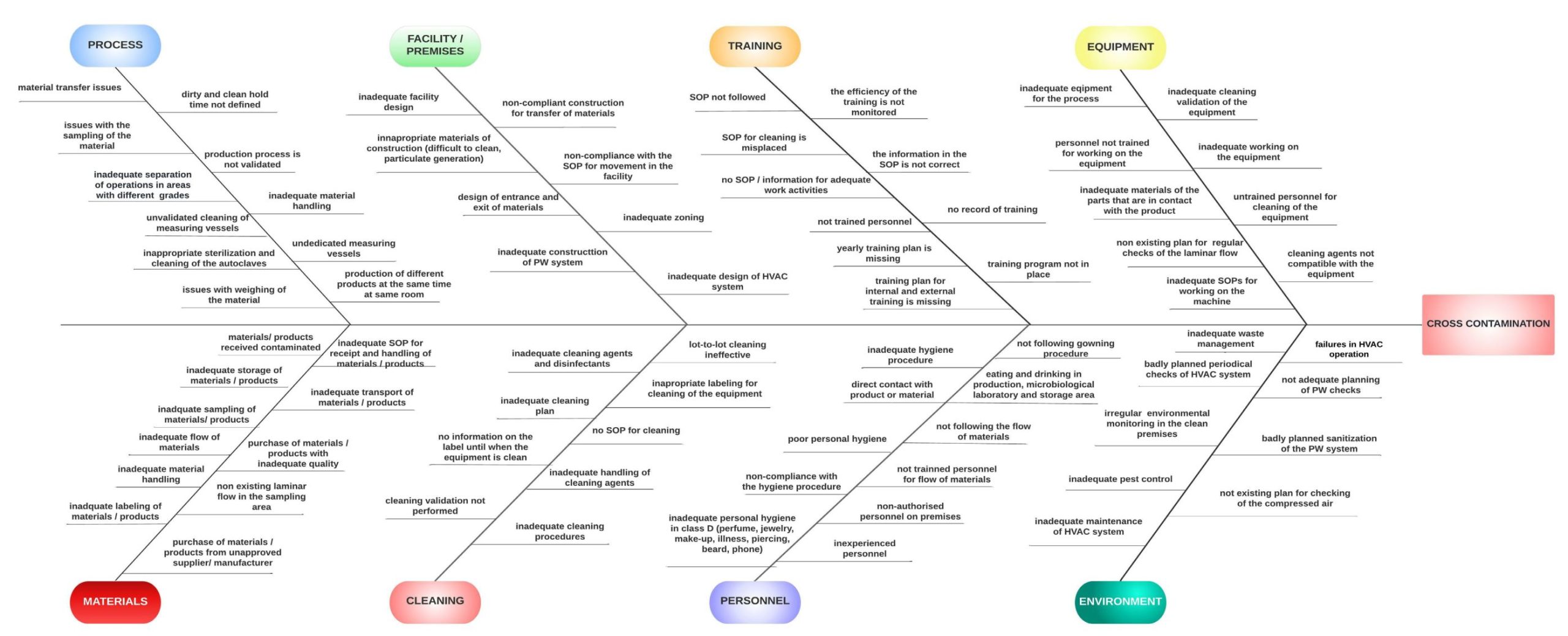
The conduction of the risk analysis is done in accordance to ICH Q9: Quality Risk Management and two tools are used: Ishikawa diagram and FMEA. Ishikawa diagram – The cause-and-effect diagram is a tool we used to identify, examine and display the possible causes of contamination. With this method we illustrated the connection between the risk (contamination) and all the factors that contribute to its occurrence.
![]()
The identified hazards for the possible occurrence of contamination are then analyzed using the tool FMEA (Failure Modes and Effect Analysis). The method takes into account the parameters probability of occurrence, severity of the risk and the possibility of its detection. The risk class is determined by the value of RPN (Risk Priority Number) which is a numerical value. RPN is determined by the formula:
Results
With Ishikawa diagram eight possible causes of contamination are shown: production process, materials, facility/premises, cleaning, training, personnel, equipment and environment. Using the FMEA tool, the root causes that could lead to contamination are analyzed and evaluated. According to their RPN value, the risks are categorized as low, medium and high risks. Total number of 308 risk were identified. Existing controls for decreasing the risk level are analyzed, through which the risks are reduced to an acceptable level that ensures that the company proactively prevent the possibility of contamination, i.e., that safe and efficient products are manufactured in accordance with the GMP principles.
Conclusion
Risk analysis was performed in order to determine the potential sources of contamination in the production facility and premises using two tools – Ishikawa diagram and FMEA. Eight main potential causes of contamination have been identified: production process, materials, facility/premises, cleaning, training, personnel, equipment and environment.
The risks were analyzed with FMEA and 308 potential root causes were identified. Measures for proactive prevention of contamination have been established, i.e. it is necessary that the facility and premises are well designed and constructed for their indented activities, the company has well-trained stuff, good design and maintenance of the HVAC system, cleaning validation is performed, the process of flow of materials is well established, as well that there is adequate documentation for all activities that guarantee safe and efficient product.
Download the full poster on “Risk-based contamination control strategy of manufacturing non-sterile pharmaceutical products” here
(click the picture to download the poster)
Source: Bionika brochure “Risk-based contamination control strategy of manufacturing non-sterile pharmaceutical products”


