Spray Dried Progesterone Formulations for Carrier Free Dry Powder Inhalation
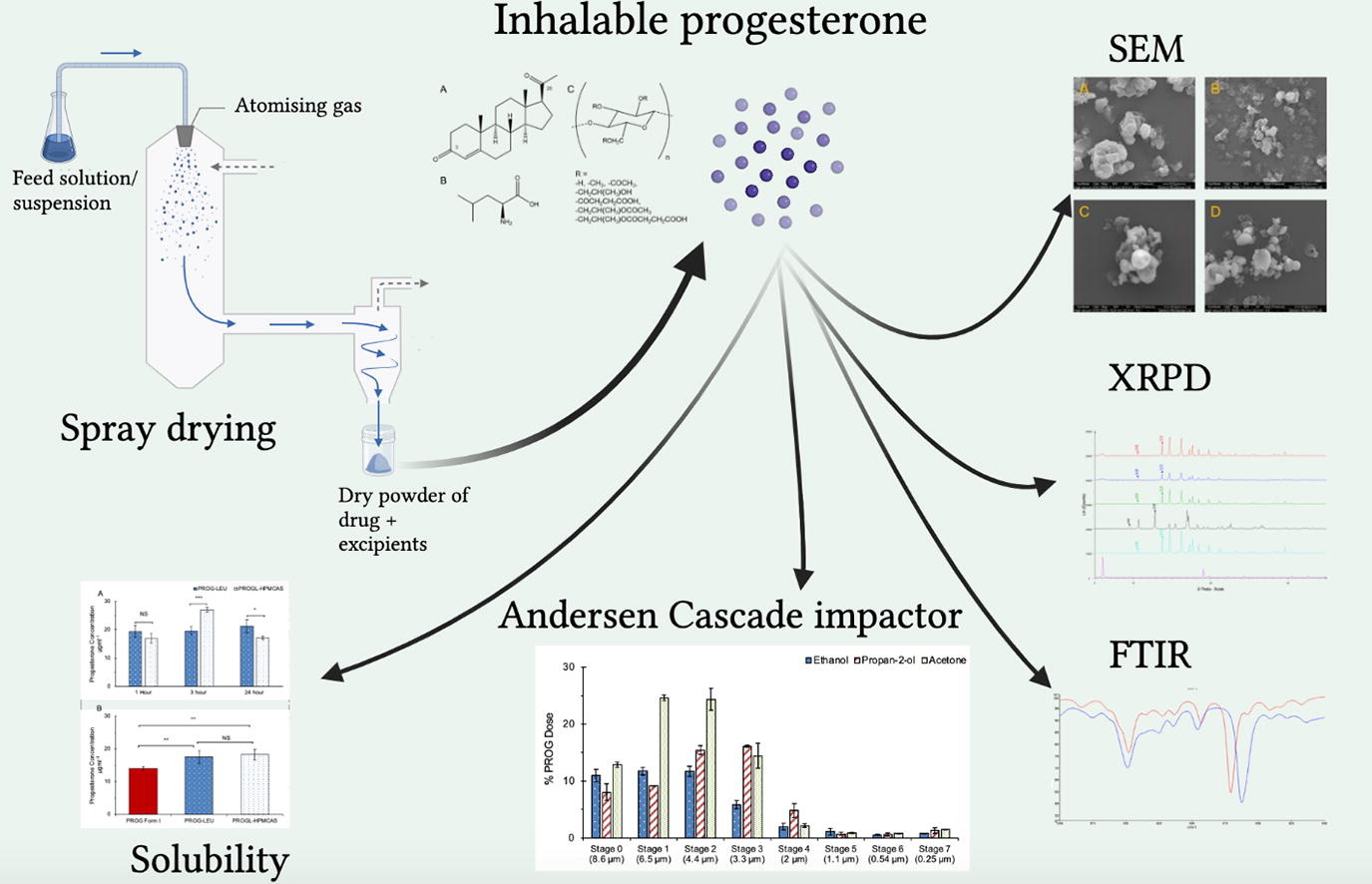
Low oral absorption and extensive first pass metabolism of progesterone is reported for many oral formulations which warrants investigation into other routes of administration. It is the aim of this study to investigate the generation of inhaled formulations of progesterone though a spray drying approach with a focus on how spray drying impacts the physicochemical properties of progesterone. Formulations of progesterone with L-leucine and hydroxypropyl methylcellulose acetate succinate (HPMCAS) are reported to this aim. X-ray diffraction, spectroscopy and thermal analysis were used to characterise these formulations and confirmed that progesterone crystallises as the Form II polymorph during spray drying regardless of the solvent used. The resultant formulations showed higher aqueous solubility than progesterone Form I starting material and the addition of HPMCAS was shown to temporarily enable a supersaturated state. Thermal analysis was used to show that the Form II polymorph was sensitive to transformation to Form I during heating. The addition of L-leucine to the formulations reduced the temperature for the polymorphic transformation by ∼10 °C. However, when HPMCAS was added to the formulation, the Form II polymorph was prevented from transforming to the Form I polymorph.
Cascade impaction was used to determine the aerosol performance of the spray dried powders and showed promising lung deposition profiles (mass median aerodynamic diameter 5 µm) with significant variation depending on the organic solvent used and the ratio of organic to aqueous phase in the feedstock. However, further optimisation of formulations was required to direct more progesterone into the alveolar regions. The addition of HPMCAS was seen to increase the alveolar deposition and therefore formed a formulation with a lower fine particle fraction and mass median aerodynamic diameter. The most suitable formulation for inhalation was formed from a 50:50 acetone:water destockck and showed an ED, FPF and FPD of 81.7%, 44.5% and 7.3 mg respectively. Therefore, HPMCAS is suggested as a suitable excipient to increase solubility, prevent polymorphic transformation and improve inhalation properties of spray dried progesterone formulations. This study highlights the use of spray drying to form inhalable progesterone powders with higher solubility which may broaden the application of this medicine.
2.1. Materials
Progesterone (PROG) and L-leucine (LEU) (Fig 1) were obtained from Sigma-Aldrich (Dorset, UK). Ethanol (EtOH), HPLC water, acetone (ACE), acetonitrile and propan-2-ol (IPA) were obtained from Fisher-Scientific Limited (Leicestershire, UK). Hydroxypropyl methylcellulose acetate succinate HG grade (HPMCAS) was obtained from Shin-Etsu Chemical Co (Tokyo, Japan) (Fig 1). Gamble’s solution was obtained from Pickering Laboratories (Mountain View, California). All chemicals were used as received.
Download the full research as PDF here: Spray Dried Progesterone Formulations for Carrier Free Dry Powder Inhalation
or read it here
Thomas Hibbard, Hannah Mitchell, Yoonha Kim, Kenneth Shankland, Hisham Al-Obaidi, Spray Dried Progesterone Formulations for Carrier Free Dry Powder Inhalation, European Journal of Pharmaceutics and Biopharmaceutics, 2023, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.06.018.
Watch our free webinar “Spray drying” here:


