Comparison between polymeric excipients using SeDeM expert system in combination with mathematical modeling and quality control tools
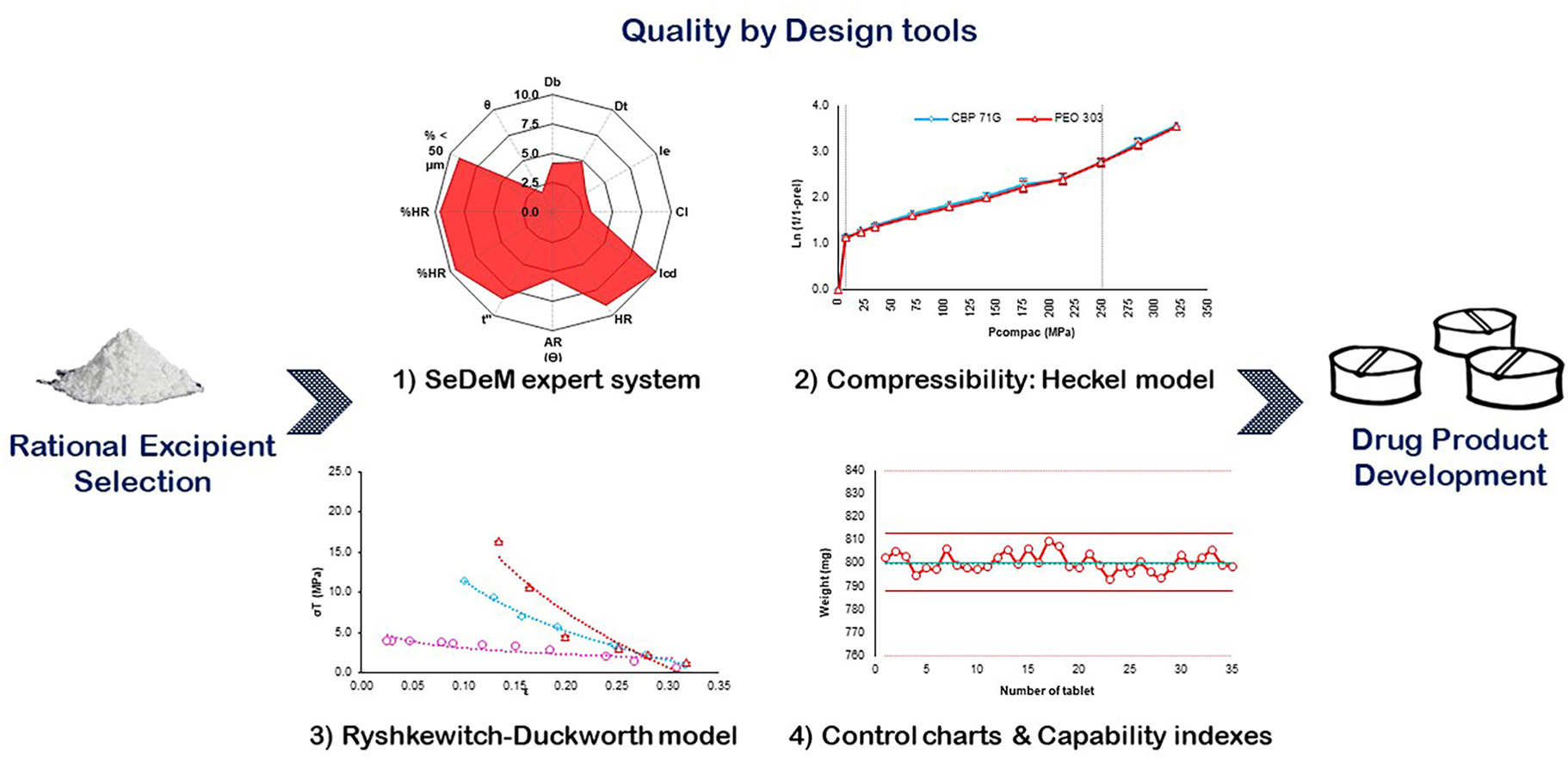
Based on the quality by design approach, we used the SeDeM expert system to assess seven polymers of different chemical nature and molecular weight for their use in direct compression. Because the SeDeM system fails by not evaluating the mechanical properties of materials, we used the Heckel and the Ryshkewitch-Duckworth mathematical models to study compressibility and compactibility of the materials, respectively. The deformation mechanism and the bonding capacity of the polymers were identified, evidencing that polyethylene oxide 301 and 303 and the carbomer homopolymer had an outstanding performance. In addition, tools such as control charts of the statistical process control and indices of the potential and actual capability in a polymer compaction process were implemented, using the weight and tablet breaking force as critical attributes of quality. In these studies, polyethylene oxide 301 and 303, hydroxypropylmethyl cellulose, and hydroxypropyl cellulose HF had the best performance and less breaking force variation. On the other hand, carbomer homopolymer exhibited the best outcomes in terms of weight variation. The use of the mentioned tools contributed to achieve a better knowledge of the properties and behavior of materials to make a rational selection of the components of a formulation.
Introduction
According to the quality by design approach, for the development of a product, a scientific approach and a risk management must be used together with other tools, which will allow generating new information of the drug product and the manufacturing process. The information and knowledge acquired during the development phase will allow to manufacture a consistent performance quality to fulfil the objective of the approach: build quality based on the design and not only on the product [1,2].
Solid state is the most common form of matter in which a substance can be, hence, this one is the most used for the development of a pharmaceutical drug product. Indeed, most formulations available in the market are solid dosage forms (capsules, tablets, or powder to be dispersed), or alternatively, their components are generally supplied in solid form. Therefore, having a deeper knowledge of the solid form at the material and product levels, and the rational selection for the solid dosage forms become critical components for the design and development of a drug product [3]. According to the Orange Book of the US Food and Drug Administration, 65% of the pharmaceutical products registered until December 2020 (39,230 registered drug products), correspond to oral solid dosage forms, and most of them are in the form of capsules and tablets [4].
Suñé Negre et al., developed a SeDeM expert system, which constitutes a systematic methodology to provide the required knowledge for the planning of the design space suggested by the pharmaceutical development guideline of the ICH (Q8 R2) and, represents an improvement to the traditional system for the physical characterization of the materials (active pharmaceutical ingredients and excipients) performed through a rheological characterization [2,5]. This system has been widely used by different authors to evaluate the performance of several materials, predict the performance of a formulation for direct compression and for the development of tablets orally dispersible by the application of different pharmacopeia tests (United States Pharmacopeia and European Pharmacopeia), as well as empirical tests [[6], [7], [8], [9], [10]]. Despite the SeDeM system evaluates the performance of a formulation materials for direct compression, it fails by not considering their mechanical properties, like the hardness of particles and the mechanism and deformation properties (elasticity, viscoelasticity, plasticity, and fragility). Therefore, it was necessary to consider the use of mathematical models, such as Heckel and Ryshkewitch-Duckworth, to study compressibility and compactibility, respectively [[11], [12], [13]]. Moreover, the variability of the materials or formulations during compression was evaluated using the weight and tablet breaking force as critical quality attributes.
Many excipients can be used for the development and manufacture of controlled release dosage forms. High molecular weight poly(ethylene oxides) (PEOs) are among the most commonly used polymers according to the literature for this purpose. PEOs are non-toxic compounds, which have high water solubility and swellability and due to their ease of production are very important for pharmaceutical industries [14]. In addition, hydroxypropyl methylcellulose (HPMC), which has the same properties, showing a high cost-effectiveness, has also been extensively used in the pharmaceutical industry [15]. Another cellulose derivative extensively used in the pharmaceutical industry is the hydroxypropyl cellulose (HPC). This polymer, as the rest of the abovementioned polymers, can form a gel layer in contact with the surrounding media, thus enabling a sustained drug release [16]. Furthermore, carbomer homopolymer (CBP), which is a high molecular weight cross-linked poly(acrylic acid) polymer, may also be used for the manufacture of oral solid dosage forms as binders or matrix tablet formers. Moreover, this poly(acrylic acid) derivative has been extensively studied as a mucoadhesive approach for drug delivery to the gastrointestinal tract [17,18]. Accordingly, physical and mechanical properties of these materials were evaluated in this work. The goal of this study was to characterize polymers commonly used in the design of modified-release drug products, such as HPMC, CBP, HPC, and PEO, at different degrees of viscosity by means of the SeDeM system, the Heckel and Ryshkewitch-Duckworth mathematical models, as well as the control charts and indices of potential and actual capacity (Cp and Cpk, respectively, for the weight and breaking force of the tablets). The latter to obtain a better knowledge of the properties of the polymers, and to substantiate the decisions for their use in the design of solid pharmaceutical forms.
Materials
The hydrophilic polymers used in this study were hydroxypropyl methylcellulose (HPMC) in two degrees of viscosity: 50 cP (HPMC 60; Sheffcel® 60HD50, Kerry, France, batch 20160262) and 15000 cP (HPMC 75; Sheffcel® 75HD15000, Kerry, France, batch 130804306) kindly donated by Chemcel SA de CV (Mexico). Carbomer homopolymer 11000 cP (CBP 71G; Carbopol® 71G, Lubrizol Co. (Wicklifffe, OH, USA batch TW75GAJ087) kindly donated by Lubrizol. Polyethylene oxide 1650–5500 cP (PEO 301; Polyox® WSR 301,
Read more
Oswaldo Castañeda Hernández, Juan Domínguez-Robles, Isidoro Caraballo, María Josefa Bernad, Luz María Melgoza Contreras, Comparison between polymeric excipients using SeDeM expert system in combination with mathematical modeling and quality control tools, Journal of Drug Delivery Science and Technology, Volume 86, 2023, 104750, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.104750.

