Evaluation the Injectability of Injectable Microparticle Delivery Systems on the Basis of Injection Force and Discharged Rate
Background
Subcutaneous injection of biopharmaceutical agents or microparticles is challenging due to issues with low injection efficiency and high residual amounts.
Objective
This study aimed to determine the important factors affecting the injectability of microparticle delivery systems, establish a suitable injection system with lower injection force and higher discharge rate, and eventually develop a reliable injectability evaluation system for injectable microparticle delivery systems in vitro and in vivo.
Methods
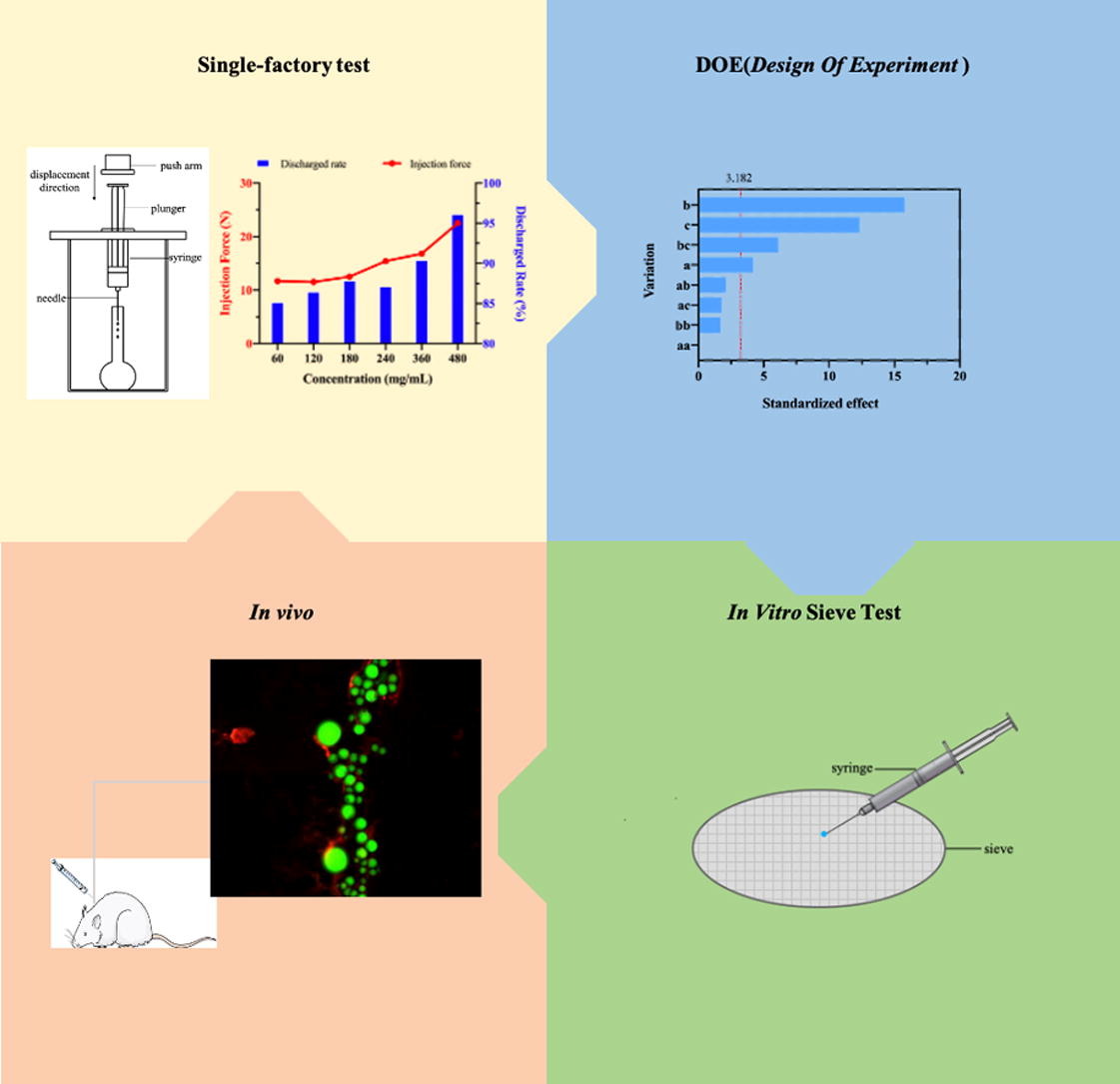
The effects of various parameters, including particle size, injection speed, concentration of microspheres suspension, vehicle viscosity, needle length and gauge were evaluated by measuring the injection force and discharge rate. The characteristics of microparticles and rheological measurement of the suspension systems were studied. A design of experiment approach was utilized to evaluate the interaction between the microsphere suspension, vehicle viscosity and needle gauges. Both in vitro sieve tests and in vivo tests in rats were conducted to evaluate injectability.
Results
The in vitro test results showed that the vehicle viscosity and injection speed have varying effects on discharge rate and injection force, respectively. Particle size and needle gauge have substantial influence on injectability, larger particle size and smaller needle gauges resulting in poor injectability, while the needle gauge was found to have the greatest influence on injectability. Levonorgestrel (LNG) microsphere and glass bead were relatively uniform spherical, the glass bead had extremely smooth surface; while mesoporous silica had irregular shape. The settling rate of glass bead was the fastest, which was about 18 times faster than the LNG microsphere. The CMC-Na had a poor interaction with the LNG microspheres, glass bead and mesoporous silica and showed basically Newtonian behavior in the shear rate range of 0.1 s-1∼ 100 s-1. When shear rate increased to more than 100 s-1, no obvious shear thinning behavior was observed. CMC-Na formed a nodule structure with whether LNG microspheres or the glass beads, which were much lower than that with the mesoporous silica in static state, among which the glass beads were the weakest. The viscosity of the suspension increased with the rising of the volume fraction of particles. Fundamentals of hydrodynamics in capillaries were referenced, such as Navier-Stokes Law equation, Krieger-Dougherty (K-D) equation, Hagen-Poiseuille equation. The best results achieved was using a suspension concentration of 120-240 mg /mL and a viscosity of 60 cP at 20°C with 23-gauge needles. The optimized conditions were verified in vivo tests. It was proven that the LNG microsphere suspension had a good injectability when injected into subcutaneous tissue of rats.
Conclusion
The injection system of injectable microparticle delivery system with lower injection force and higher discharge rate was established and the evaluation method was suitable for the injectability evaluation both in vivo and in vitro. Improved injectability would promote the clinical translation of microparticle delivery systems.
Materials
Glass beads with the mean diameter of 50 μm were provided by Shanghai Institute of Measurement and Testing Technology (Shanghai, China). Mesoporous silica (SYLOID XDP 3050) with the mean diameter of 50 μm were kindly gifted by Grace Trading (Shanghai, China) Ltd., LNG microspheres were prepared in our laboratory with the mean diameter of 50 μm. 1 mL and 2 mL syringe with Luer-Lok™ were purchased from Shanghai Kindly Medical Instruments Co. Ltd (, Shanghai, China). Needles of different size,
Read more
Chuncao Zhao, Zhihan Zhu, Xingchen Cao, Feng Pan, Fang Li, Man Xue, Yilin Guo, Yanhong Zhao, Jia Zeng, Yu Liu, Ziyi Yang, Yan Liu, Fuzheng Ren, Linglin Feng, Evaluation the Injectability of Injectable Microparticle Delivery Systems on the Basis of Injection Force and Discharged Rate, European Journal of Pharmaceutics and Biopharmaceutics, 2023, ISSN 0939-6411,
https://doi.org/10.1016/j.ejpb.2023.06.017.
Read more on “Mesoporous Silica” here:


