Prediction of dissolution performance of uncoated solid oral dosage forms via optical coherence tomography
Abstract
Real-time prediction of the dissolution behavior of solid oral dosage forms is an important research topic. Although methods such as Terahertz and Raman can provide measurements that can be linked to the dissolution performance, they typically require a longer time off-line for analysis. In this paper, we present a novel strategy for analyzing uncoated compressed tablets by means of optical coherence tomography (OCT). Using OCT, which is fast and in-line capable, makes it possible to predict the dissolution behavior of tablets based on images. In our study, OCT images were obtained of individual tablets from differently produced batches. Differences between tablets or batches in these images were hardly visible to the human eye.
Advanced image analysis metrics were developed to quantify the light scattering behavior captured by the OCT probe and depicted in the OCT images. Detailed investigations assured the repeatability and robustness of the measurements. A correlation between these measurements and the dissolution behavior was established. A tree-based machine learning model was used to predict the amount of dissolved active pharmaceutical ingredient (API) at certain time points for each immediate-release tablet. Our results indicate that OCT, which is a non-destructive and real-time technology, can be used for in-line monitoring of tableting processes.
Introduction
Solid oral dosage forms are one of the most important pharmaceutical products. A key criterion of their efficacy is the absorption of the active pharmaceutical ingredient (API) inside the human body. One main factor that influences the API absorption is the dissolution behavior, which is largely affected by the properties of the material (solubility, excipients, etc.) used in the production process and the process parameters (compression force, etc.). As a key indicator of product performance, it is considered a critical quality attribute (CQA).
In the case of coated tablets, a functional coating regulates the dissolution behavior to achieve a desired target dissolution profile by varying the coating material’s attributes and the coating layer’s thickness. For uncoated tablets, the material composition and structure, the solubility, the particle properties and the process conditions are the determining factors influencing the dissolution behavior. Also, residual moisture and tablet hardness are important[1].
Traditionally, dissolution is tested in analytical labs via methods that have to be specifically developed for each formulation. Typically, six or twelve tablets are dissolved in individual vessels, and the samples are collected at a specific time and quantified using an appropriate analytical method. Details regarding analytical dissolution method development can be found in USP42-NF37 <711> [2]. From these measurements, a dissolution profile is determined. However, this procedure is labor-, time- and cost-intensive. To obtain a good representation of an average dissolution behavior of the tablets in a batch, large sample sizes would be desirable. Established dissolution testing does not allow for a larger quantity of tablets to be inspected. Hence, surrogate methods are of interest to measure attributes that can be linked to dissolution.
Once the tablets are ingested the first step is disintegration, followed by dissolution of the granules or primary particles. Clearly, penetration of the gastric fluid into the porous structure of the tablet is a rate-determining step for disintegration. One step towards an understanding of disintegration and the dissolution behavior is to analyze the internal structure of the tablet, i.e., the porosity or the pore size distribution (PSD). The established way of measuring porosity and PSD is in off-line mode via gas adsorption. In the BET method[3] typically nitrogen or krypton adsorption is used to measure the specific surface area and PSD. Additional approaches for specific surface area have been developed and investigated. Other methods of investigating the PSD include mercury porosimetry[4], [5] and helium pycnometry[5]. Another gas-based way to quantify porosity is gas-in-scattering-media-absorption spectroscopy (GASMAS)[6], [7], [8]. Other techniques of assessing porosity rely on waves that hit the tablet and on analyzing the absorption and reflection behavior, e.g., Raman spectroscopy[9], [10], [11] and X-ray microtomography[10]. In the last decade, Terahertz-based spectroscopy has been successfully applied to determine tablet porosity[12], [13], [14], [15]. In particular, Terahertz measurements of porosity have been correlated with the dissolution performance[14], [16]. However, none of these methods have been applied in-line or in real-time. Methods, such as NIR, have been used to contribute to the real-time prediction of dissolution[17] but only in combination with other process and sensor data.
In optical coherence tomography (OCT), a spectrum is obtained via interferometry, in which a light beam is split into two parts, one is reflected by a reference mirror, the other one by the sample to measure. The spectrum is then calculated by comparing these two reflected light beams, from which images are computed and analyzed. In the last few years, OCT has provided insights into the coating quality attributes of certain coated solid oral dosage forms, such as tablets and pellets[18], [19], [20]. In particular, the coating thickness measured via OCT has been correlated with dissolution performance[21]. In OCT, the light from the probe penetrates the coating layer, and the interface between the air and the coating and between the coating and the core is depicted in images, which can subsequently be automatically evaluated using machine learning[18], [22], [23] to obtain the thickness values and other coating layer attributes, such as the surface roughness and homogeneity. It has been demonstrated that OCT can be installed in-line inside a coater to obtain live, real-time measurements[24]. Ideally, such measurements can be used to support the coating process and, eventually, a real-time release testing (RTRT) strategy.
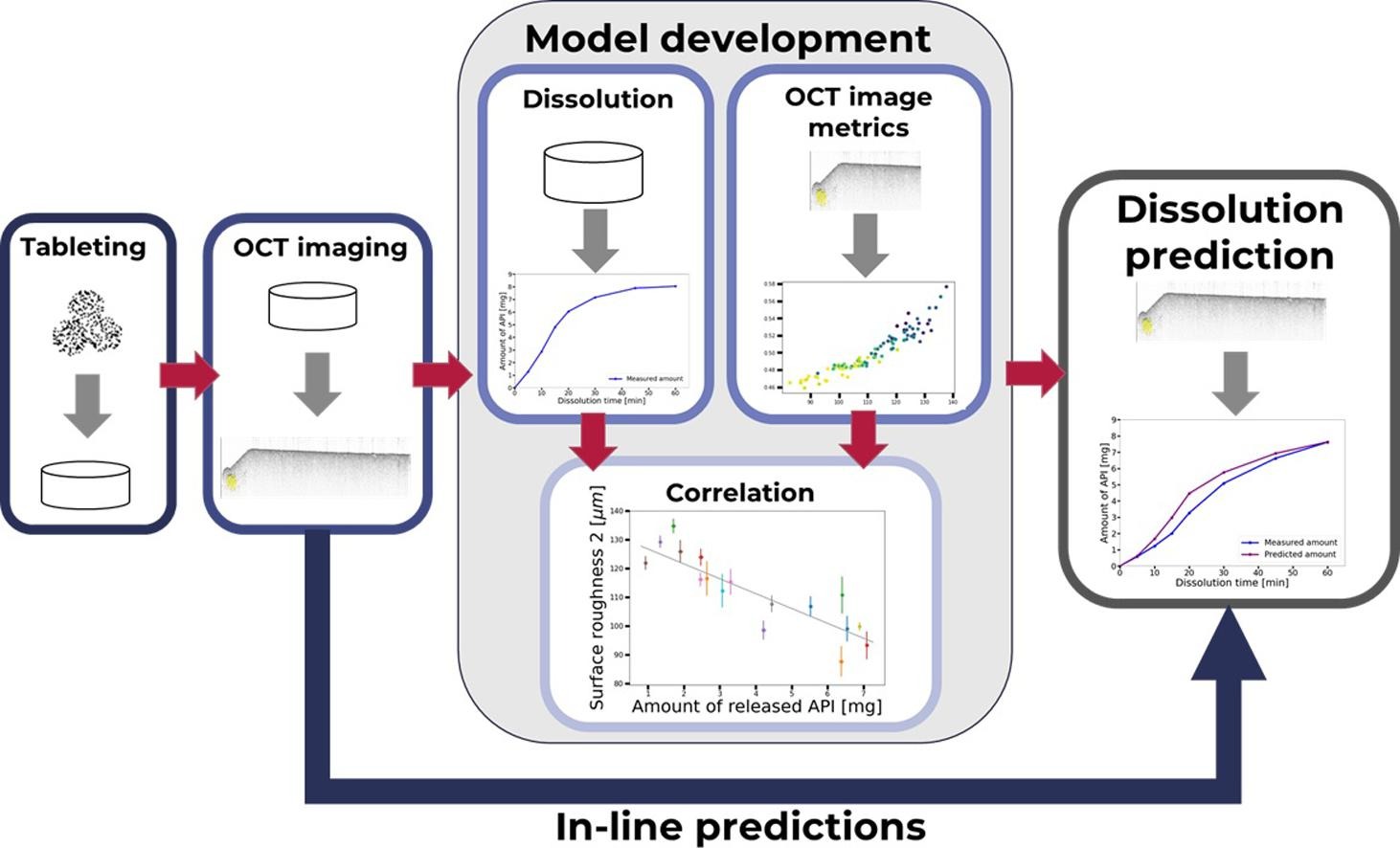
In this work, we used OCT data to assess the dissolution behavior of uncoated tablets. Recently developed OCT image evaluation strategies[22] allow to calculate quantitative attributes of OCT images of tablets that do not show a coating layer in the images. In particular, some of these attributes quantify the surface topography and inner morphology of the region about 100 – 200 µm– below the surface of the tablet. These evaluation strategies were used to establish a strong link between the OCT image metrics and the dissolution behavior of the tablet via a tree-based machine learning model. This novel approach enables the prediction of dissolution behavior based on real-time data generated by a single in-line monitoring tool.
Read more here
Material
Tablets were produced in 17 batches with different process settings (Table 1). From each batch, six individual tablets were measured via OCT using the system OSeeT Pharma 1D (Phyllon GmbH, Austria).
Elisabeth Fink, Selma Celikovic, Jakob Rehrl, Stephan Sacher, Jesús Alberto Afonso Urich, Johannes Khinast, Prediction of dissolution performance of uncoated solid oral dosage forms via optical coherence tomography, European Journal of Pharmaceutics and Biopharmaceutics, Volume 189, 2023, Pages 281-290, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.07.003.
Read more on OCT Phyllon “Optical Coherence Tomography as a novel tool for non-invasive membrane thickness monitoring of co-extruded drug-delivery systems” here: