Fast time-resolved micro-CT imaging of pharmaceutical tablets: Insights into water uptake and disintegration
Abstract
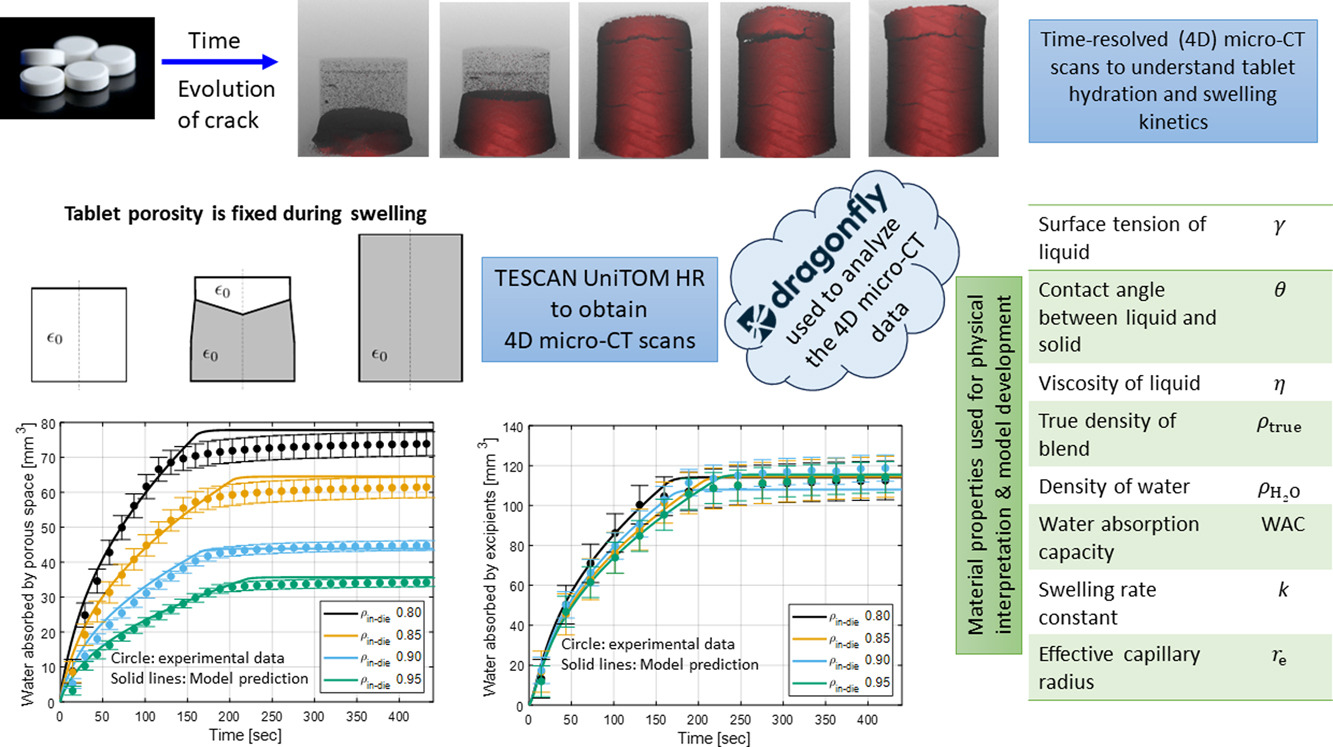
We use dynamic micro-computed tomography (micro-CT) with a high temporal resolution to visualize water penetration through the porous network of immediate-release pharmaceutical solid tablets and characterize dynamic swelling and disintegration mechanisms. We process the micro-CT images using two theoretical scenarios that reflect different paths of pore structure evolution: a scenario where tablet porosity remains constant during the swelling process and a scenario where the tablet porosity progressively diminishes and eventually closes during the swelling process. We calculate the time evolution of the volume of water absorbed by the tablet and, specifically, absorbed by the excipients and the pore structure, as well as the formation and evolution of cracks.
In turn, the three-dimensional disintegration pattern of the tablets is reconstructed. Restricting attention to the limiting scenario where tablet porosity is assumed fixed during the swelling process, we couple liquid penetration due to capillary pressure described by the Lucas–Washburn theory with the first-order swelling kinetics of the excipients to provide a physical interpretation of the experimental observations. We estimate model parameters that are in agreement with values reported in the literature, and we demonstrate that water penetration is dominated by intra-particle porosity rather than inter-particle porosity.
Introduction
The most common orally administered solid unit dosage form for drug delivery is compacted tablets. The main advantages encompass accurate control of dosing, easy storage, cost-effectiveness, and easy administration. Tablet formulations generally consist of active pharmaceutical ingredients (APIs) and a mix of inactive ingredients, termed excipients, which must be physiologically inert (Desai et al., 2016). Excipients are critical to the tablet design, determining its functionality and performance. Controlling the release of API at the drug plasma level leads to reduced side effects while providing greater patient compliance and convenience (Abdul and Poddar, 2004). To achieve the desired release profile, one needs to have a well-researched view of API’s fundamental physicochemical and biopharmaceutical properties, appropriate excipient selection, and manufacturing condition (Wen and Park, 2011). Different kinds of excipients, including disintegrants, fillers, binders, glidants, lubricants, antioxidants, ultraviolet absorbers, dissolution modifiers, absorbents, flavoring, agents, colorants, wetting agents, and preservatives may be added to the formulation (Kottke and Rudnic, 2002, Augsburger et al., 2007). For example, an excipient such as a soluble or insoluble filler (lactose/mannitol vs. microcrystalline cellulose) added to a formulation may affect the drug release rate in different ways (Rosiaux et al., 2014).
Different complex physical and chemical phenomena induce drug release from a pharmaceutical system, and a mathematical model to describe these mechanisms is difficult to find (Costa and Lobo, 2001). According to Siepmann and Peppas (2012), in many cases, simple empirical and semi-empirical models are sufficient to understand drug release mechanisms and design a new product. For example, Ferdoush and Gonzalez (2023) developed semi-mechanistic models to predict changes in dissolution profiles due to manufacturing process disturbances and studied how these models can enable real-time release testing. However, detailed information and complex mechanistic theories must be applied in many cases. According to Peppas and Narasimhan (2014), models that capture the mechanistic theory will provide new insights into the release mechanism when combined with precise experimental observations. Thus, realizing that the underlying coupled mechanics of imbibition, swelling, disintegration, and dissolution are still an open question. Specifically, the following dominant mechanisms of disintegration are identified (Quodbach and Kleinebudde, 2016, Markl and Zeitler, 2017, Faroongsarng and Peck, 1994, Moreton, 2008, Desai et al., 2016): (i) liquid intake through the open porous network by capillarity action, (ii) liquid transport from/to the pore-space network to/from the particles, (iii) liquid transport from particle to particle facilitated by the contact interfaces and solid bridges created during the compaction process, (iv) swelling of the particles due to liquid penetration, (v) deformation of the granular structure to relax internal stresses created during swelling, (vi) softening of particles and solid bridges due to liquid uptake, and (vii) breakage of solid bridges due to excessive relaxation of internal stress and mechanical softening. Characterizing these processes at the microstructure level is challenging because one needs to visualize them from the inside out. Another challenge lies in identifying the time scale of these processes, as sample relaxation or other processes can happen in seconds to minutes. Time-resolved micro-computed tomography (micro-CT) or 4D CT, where time is defined as the fourth dimension, is an excellent non-destructive technique to visualize these mechanisms (Boever et al., 2021).
During a time-lapse imaging process, a series of individual scans is taken over a specific experiment time period, often referred to as ‘interrupted 4D’ experiments. Conversely, dynamic tomography does not incorporate any delays between individual scans. Instead, an uninterrupted acquisition approach is adopted during the scan time interval of the entire process in examination (Dewanckele et al., 2020). Dynamic micro-CT is a powerful imaging technique that can provide high temporal resolution, improved contrast, reduced motion artifacts, and accurate 3D reconstruction where no crucial information is lost. These advantages make it ideal for studying rapid dynamic processes in materials science, biology, and engineering, among other fields. However, time-lapse micro-CT is still useful for studying slower dynamic processes and changes that occur gradually and consistently over longer time scales (Hunter and Dewanckele, 2021, Dewanckele et al., 2021, van der Wal, 2021) such as corrosion of metals, creep processes, or slow crystallization phenomena (Boever et al., 2021). TESCAN, a leading global producer and supplier of scanning electron microscopes, has designed and manufactured the first dynamic micro-CT system to offer both sub-micron spatial resolution and high temporal resolution in a single system (Vacuum, 2022). Specifically, the TESCAN UniTOM HR’s high-resolution imaging capabilities make it a valuable tool for material characterization and failure analysis (Joseph et al., 2023, Pinto et al., 2023, Mesquita et al., 2022, Butenegro et al., 2022, Javanshour et al., 2023). However, these 4D micro-CT capabilities have yet to be used to quantify fast structural changes in pharmaceutical solid tablets and understand disintegration behavior (cf., quantitative studies of total volume increase of tablets or individual grains Rudnic et al., 1982).
In this study, a TESCAN UniTOM HR is used to visualize (i) penetration of water inside the tablet through the porous network and (ii) disintegration dynamics of immediate-release tablets, i.e., tablets that disintegrate and completely dissolve upon exposure to water in a few minutes (see, e.g., Markl and Zeitler (2017)). This rapid disintegration poses challenges for slower, time-lapse, or interrupted micro-CT procedures since water uptake, swelling, and disintegration processes cannot be paused during the experiment. We have also investigated the pore structure changes and estimated the time evolution of total tablet volume during swelling. Furthermore, we will provide a physical interpretation of the results obtained from these micro-CT images by identifying the water pathways as it fills the pore structure and it is absorbed by the swelling excipients (Hamraoui and Nylander, 2002, Washburn, 1921, Schott, 1992a, Schott, 1992b).
The paper is organized as follows. Section 2 discusses materials for fabricating the tablets used in this study and methods for obtaining the dynamic micro-CT images. Microstructure reconstruction and segmentation process are also described in the same section. The micro-CT image processing results are presented in Section 3. Physical interpretation of the results is provided in the same section. Concluding remarks are presented in Section 4.
Read more here
Shumaiya Ferdoush, Sarah Bu Kzam, Pedro H.C. Martins, Jan Dewanckele, Marcial Gonzalez, Fast time-resolved micro-CT imaging of pharmaceutical tablets: Insights into water uptake and disintegration, International Journal of Pharmaceutics, Volume 648, 2023, 123565, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123565.
Read more on Disintegrants – Pharmaceutical Excipients here:


