Ensuring the quality of 3D printed medicines: Integrating a balance into a pharmaceutical printer for in-line uniformity of mass testing
Abstract
Semi-solid extrusion (SSE) 3D printing has great potential to be integrated in a clinical setting, with the use of pre-filled and disposable pharma-ink syringes meeting regulatory good manufacturing practice (GMP) requirements. Uniformity of mass testing is a critical quality attribute and is carried out by weighing a specific amount of dosage units in a single batch and finding the average mass to evaluate any deviations. However, this test for small batches of 3D printed medicines may require weighing the entire manufactured batch. To overcome this limitation, an in-line analytical balance was implemented inside a GMP pharmaceutical 3D printer, with a specialised software-controlled weighing system for the automated mass uniformity testing of the entire printed batch. Three different dose batches (n = 28) of hydrocortisone pharma-ink were 3D printed and subjected to in-line mass uniformity testing. The developed software was capable of registering the weights of all individual printlets and accurately detecting any deviations within the accepted limits. Only one printlet was outside the accepted weight range, a result of the first print often being imperfect due to the semi-solid nature of the pharma-ink. The weight results were compared against an external analytical balance, and no significant differences were found. This study is the first to integrate an analytical balance inside a pharmaceutical printer, automating the dosage form mass uniformity testing which can save time, labour, and resources, whilst improving the quality control testing of 3D printed pharmaceuticals.
Introduction
Additive manufacturing, also known as three-dimensional (3D) printing (3DP), involves the production of tailor-made 3D structures through digital computer-aided design (CAD) files, in a layer-by-layer manner [[1], [2], [3]]. 3DP has been widely investigated in the pharmaceutical field to produce unique and personalised medicines in the form of Printlets (3D printed tablets) [[4], [5], [6], [7]]. Pharmaceutical 3DP can manufacture medicines in a wide range of doses, shapes, flavours, colours, drug combinations and drug release profiles, unique to each patient or disease state [[8], [9], [10], [11]]. Such features make this technology favourable to numerous patient groups such as paediatrics and geriatrics, polypharmacy patients, and rare disease patients. 3DP has also been investigated as an alternative to pharmaceutical compounding, providing treatment at the point-of-care [12]. The use of 3DP would ultimately reduce dosing errors and contamination, improve the quality, and automate and accelerate the compounding process [[13], [14], [15]].
Among the various 3DP technologies investigated, material extrusion technologies are the most used in pharmaceutics [16]. The main extrusion-based technologies are fused deposition modelling (FDM) [[17], [18], [19]], direct powder extrusion (DPE) [[20], [21], [22]], and semi-solid extrusion (SSE) [[23], [24], [25]], depending on the nature of the pharma-ink (drug loaded ink – term coined for the first time by Ref. [26]). SSE 3DP uses semi-solid pharma-inks, in the form of gels or pastes, that are deposited layer-by-layer to create a solid dosage form [[27], [28], [29], [30], [31]]. Printing can be carried out at low temperatures, making it especially beneficial for bioprinting applications or thermolabile drugs [[32], [33], [34]]. SSE is capable of producing unique dosage forms, such as chewable tablets [35,36], orodispersible films (ODFs) [37,38], medical devices [39,40], or polypills [9]. The use of Generally Recognized As Safe (GRAS) materials, no contamination through the use of disposable syringes and printheads, and easy system maintenance and cleaning are key to meeting regulatory good manufacturing practice (GMP) requirements [41]. As a result, this has been the first 3DP technology to be used in a clinical study for paediatric patients with maple syrup urine disease (MSUD) [42], and a bioequivalence study investigating 3D printed sildenafil citrate [43].
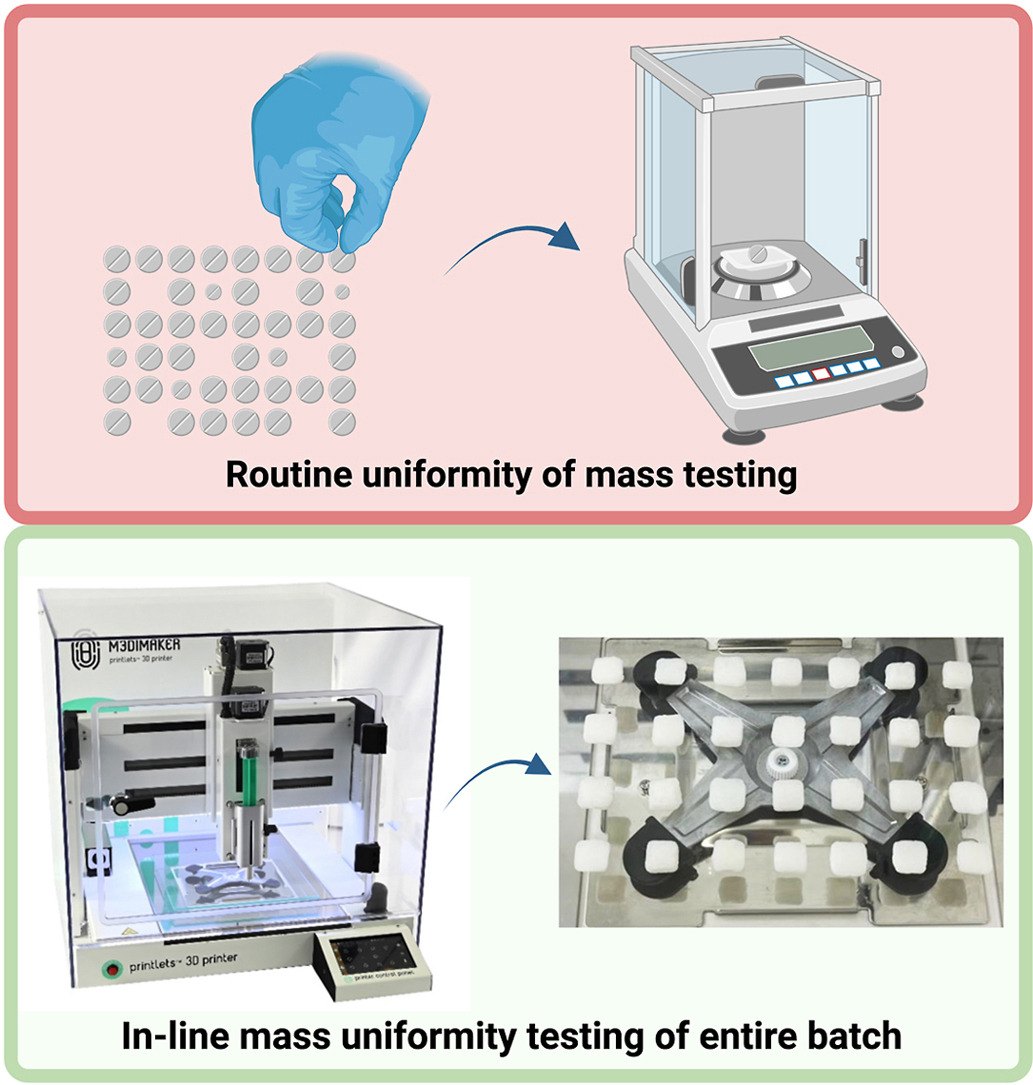
To prove that 3DP at the point-of-care is a feasible approach to personalised medicine manufacture, rigorous quality control (QC) measures must be in place to ensure optimal performance and product safety [44]. The European Pharmacopeia (Ph. Eur.) and United States Pharmacopeia (USP) have strict requirements for uniformity of dosage units, such as uniformity of content and uniformity of mass [45]. In general, conventional uniformity of mass testing for tablets in the pharmaceutical industry involves the weighing of 20 dosage forms at random from the prepared large batch and determining the average mass to confirm if any deviate from that value by a predetermined percentage. For example, in the case of tablets with an average mass of ≥250 mg, the accepted limit is 5%. This is a destructive procedure since the 20 units are removed from the batch and analysed in the QC laboratory before acceptance. As an industrial process control measure, some of the tablets prepared during the compression process are weighed in real time and the weight and deviation are recorded on the control chart. However, 3DP technology is intended to prepare small and personalised batches of medicines on demand for specific patients at the point-of care, therefore, the uniformity of mass testing may require weighing of the whole batch. With the implementation of a balance within the pharmaceutical 3D printer, mass uniformity testing of the whole printed batch could be carried out in-line. Compared to the current Ph. Eur. and USP requirements, this automatic method would control the weight of all the printlets in the batch. The use of a specialised software linked to the balance can also provide quick information on the mass of each printlet and any deviations that occur during printing. Automating the process can save time, labour and resources, whilst improving the QC testing of 3D printed pharmaceuticals.
Conventional QC measures for drug products involve quality by testing (QbT), which are often destructive in nature and include labour intensive and time-consuming techniques such as high-performance liquid chromatography (HPLC) [46]. Performing this type of testing at the point-of-care would incur higher costs in terms of time and resources as the batches produced are small and personalised for each patient. An alternative approach to QC for 3D printed dosage forms is quality by design (QbD), which involves the identification of critical quality attributes (CQAs) during manufacture. Process analytical technology (PAT) tools are emerging as techniques for analysing the key CQAs, which can be installed in-line, on-line, at-line, or off-line, supplying both qualitative and quantitative information. Examples of PAT tools already investigated with 3DP include an integrated pressure sensor in an SSE printhead which could characterise the rheological properties of the pharma-ink during printing and inform the user of the system performance [47]. Another example of integrated PAT tools is the use of near-infrared (NIR) spectroscopy for drug quantification in two-dimensional (2D) and 3D printed pharmaceuticals [26,[48], [49], [50], [51], [52], [53]], which can be used to conduct automated and non-destructive uniformity of content tests at the point-of-care [26].
This study aimed to integrate an in-line analytical balance into a GMP pharmaceutical 3D printer and establish a software-controlled weighing procedure to enable automated mass uniformity testing. A semi-solid pharma-ink containing hydrocortisone was developed and validated using the integrated balance and 3D printer software. Three batches of printlets (n = 28) with varying doses were 3D printed and subjected to in-line mass uniformity testing. The results of these tests were compared against those obtained from an external analytical balance. Finally, the printlets were comprehensively characterised in terms of drug content, in vitro drug release, and physicochemical properties.
Download the full article as PDF here: Ensuring the quality of 3D printed medicines
or read it here
Materials
Hydrocortisone base (MW 362.46 g/mol, Acofarma, Barcelona, Spain), Gelucire 50/13 pellets (Gattefossé, SAS, Saint-Priest, France) and glycerol anhydrous (≥99.0%, Merck, Darmstadt, Germany) were used for the preparation of the pharma-ink. Acetonitrile and methanol (HPLC grade, Sigma-Aldrich, Dorset, UK) were used as the mobile phase for the drug content assay. Hydrochloric acid (37%, Ph. Eur, Scharlau, Barcelona, Spain) was used for the preparation of the dissolution testing media. All materials were used as received.
Carlos Bendicho-Lavilla, Lucía Rodríguez-Pombo, Patricija Januskaite, Carlos Rial, Carmen Alvarez-Lorenzo, Abdul W. Basit, Alvaro Goyanes, Ensuring the quality of 3D printed medicines: Integrating a balance into a pharmaceutical printer for in-line uniformity of mass testing, Journal of Drug Delivery Science and Technology, Volume 92, 2024, 105337, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105337.
Read more on “3D Printing” here:


