Development of novel portable NIR spectroscopy process analytical technology (PAT) tool for monitoring the transition of ibuprofen to ibuprofen sodium during wet granulation process
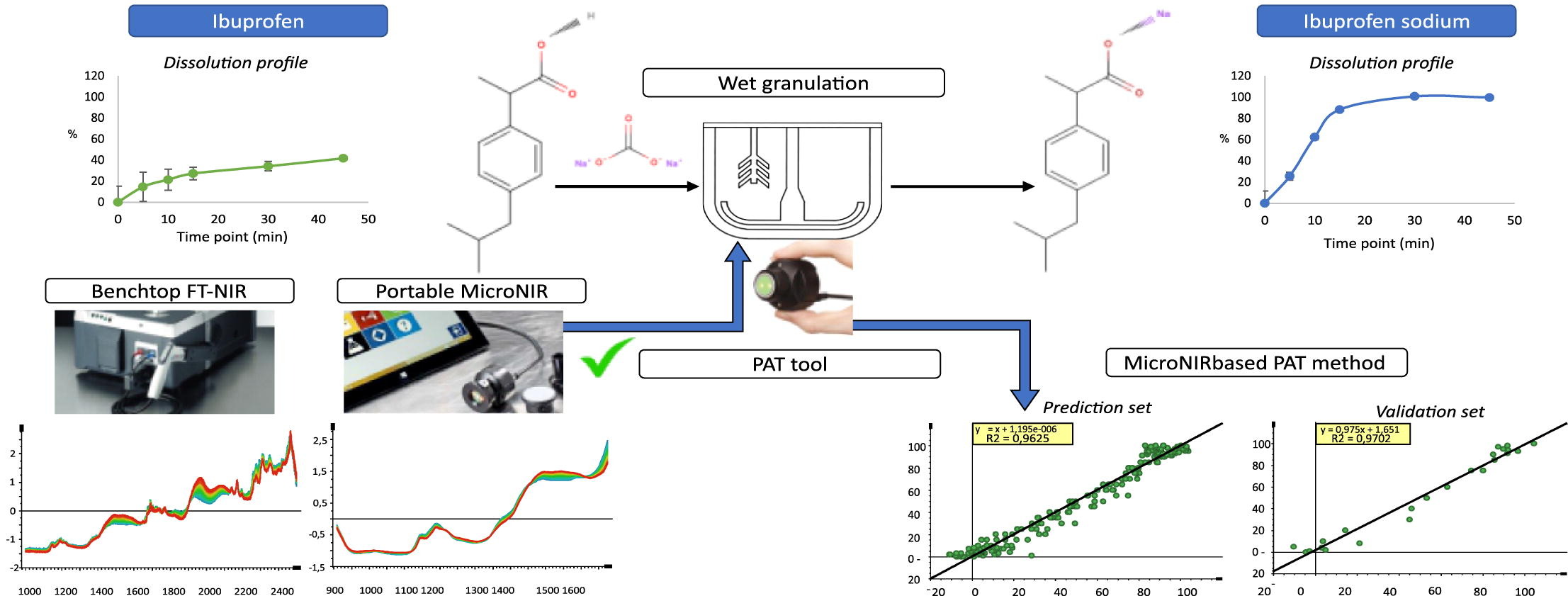
The aim of this research was to develop a process analytical technology (PAT) tool for monitoring the transformation process of the active ingredient ibuprofen into the fast-dissolving salt ibuprofen sodium during the wet granulation process. Two near-infrared (NIR) spectrophotometers, namely portable and benchtop spectrophotometer, were compared. During the analysis with the built models, both demonstrated comparable accuracy and precision (R2X = 0.995, R2Y = 0.927, Q2 = 0.995, and R2X = 0.99, R2Y = 0.948, Q2 = 0.992, respectively).
Highlights
- A NIR based PAT tool for monitoring transformation of ibuprofen to ibuprofen sodium.
- PLS models for quantification of ibuprofen conversion during wet granulation.
- Model validation on trial batches with varying ibuprofen acid-salt conversion rate.
- Dissolution rates match NIR-predicted ibuprofen sodium concentrations post-granulation.
Considering the applicability, a model based on the portable NIR spectroscopic data was chosen for further development and application as a PAT tool for monitoring different steps during the wet granulation process. The evaluation of the model’s predictive capability involved analyzing laboratory trial batches with varying amounts of sodium carbonate, resulting in different concentrations of ibuprofen sodium at the end of the wet granulation process. Subsequently, tablets were manufactured from each trial batch, followed by dissolution analysis.
The dissolution rate assays were in good agreement with the NIR-predicted concentrations of ibuprofen sodium at the end of the wet granulation process. Based on the results, the proposed model provides an excellent tool to monitor the ibuprofen acid-salt transformation process, to determine the end-point of the reaction, and to efficiently control the wet granulation process.
Read more here
Materials
Ibuprofen and ibuprofen sodium dihydrate were procured from BASF Corporation in Bishop, Texas, while sodium carbonate (fine powder) was sourced from Dr. Paul Lohmann GmbH & Co. KGaA in Emmerthal, Germany. Microcrystalline cellulose, specifically Avicel® PH 101 and Avicel® PH 102, along with croscarmellose sodium (Ac-Di-Sol), were acquired from DuPont Nutrition in Cork, Ireland. Lactose monohydrate 100 was purchased from MEGGLE GmbH & Co. KG in Germany. Colloidal silicon dioxide (Aerosil® 200)
Elizabeta Atanaskova, Veronika Angelovska, Marina Chachorovska, Natasha Anevska Stojanovska, Gjorgji Petrushevski, Petre Makreski, Nikola Geskovski, Development of novel portable NIR spectroscopy process analytical technology (PAT) tool for monitoring the transition of ibuprofen to ibuprofen sodium during wet granulation process,
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2024, 124369, ISSN 1386-1425, https://doi.org/10.1016/j.saa.2024.124369.
Read also our introduction article on Microcrystalline Cellulose here:


