Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection
Would Nanocarriers Complement Biofilm Disruption or Pave Its Road?
Introduction: Cystic fibrosis (CF) is associated with pulmonary Pseudomonas aeruginosa infections persistent to antibiotics.
Methods: To eradicate pseudomonal biofilms, solid lipid nanoparticles (SLNs) loaded with quorum-sensing-inhibitor (QSI, disrupting bacterial crosstalk), coated with chitosan (CS, improving internalization) and immobilized with alginate lyase (AL, destroying alginate biofilms) were developed.
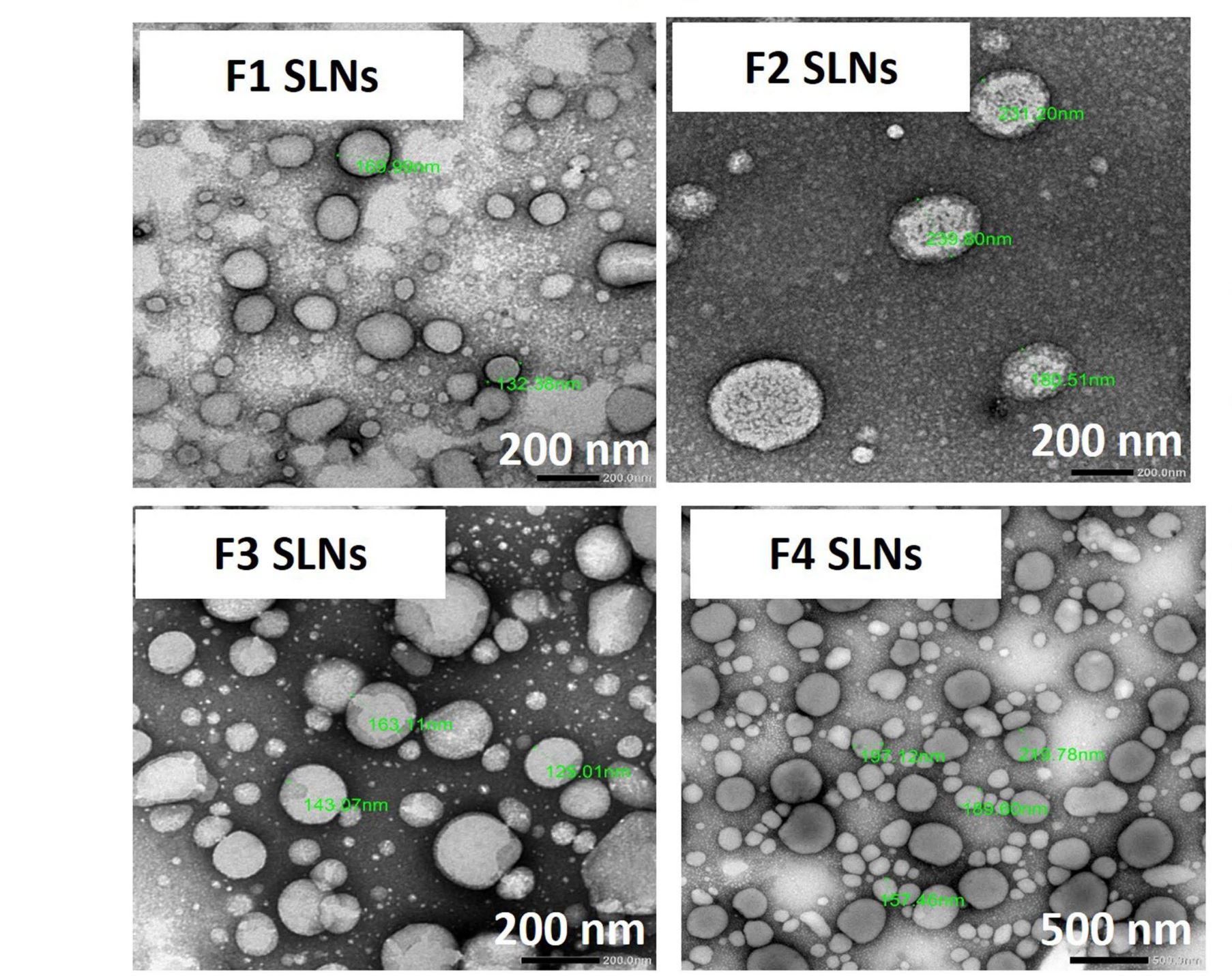
Results: SLNs (140– 205 nm) showed prolonged release of QSI with no sign of acute toxicity to A549 and Calu-3 cells. The CS coating improved uptake, whereas immobilized-AL ensured > 1.5-fold higher uptake and doubled SLN diffusion across the artificial biofilm sputum model. Respirable microparticles comprising SLNs in carbohydrate matrix elicited aerodynamic diameters MMAD (3.54, 2.48 μm) and fine-particle-fraction FPF (65, 48%) for anionic and cationic SLNs, respectively. The antimicrobial and/or antibiofilm activity of SLNs was explored in Pseudomonas aeruginosa reference mucoid/nonmucoid strains as well as clinical isolates. The full growth inhibition of planktonic bacteria was dependent on SLN type, concentration, growth medium, and strain. OD measurements and live/dead staining proved that anionic SLNs efficiently ceased biofilm formation and eradicated established biofilms, whereas cationic SLNs unexpectedly promoted biofilm progression. AL immobilization increased biofilm vulnerability; instead, CS coating increased biofilm formation confirmed by 3D-time lapse confocal imaging. Incubation of SLNs with mature biofilms of P. aeruginosa isolates increased biofilm density by an average of 1.5-fold. CLSM further confirmed the binding and uptake of the labeled SLNs in P. aeruginosa biofilms. Considerable uptake of CS-coated SLNs in non-mucoid strains could be observed presumably due to interaction of chitosan with LPS glycolipids in the outer cell membrane of P. aeruginosa.
Conclusion: The biofilm-destructive potential of QSI/SLNs/AL inhalation is promising for site-specific biofilm-targeted interventional CF therapy. Nevertheless, the intrinsic/extrinsic fundamentals of nanocarrier–biofilm interactions require further investigation.
Download the full article as PDF here: Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection
or read it here
Materials
The quorum-sensing inhibitor (QSI) used in this study was previously reported as compound 3 by Lu et al.23 It is an alkylquinolone-derived inverse agonist of the QS receptor PqsR with the IUPAC name 2-heptyl-6-nitro-4-oxo-1,4-dihydroquinoline-3-carboxamide and has been synthesized and characterized according to previously reported procedures.23,32 Gelucire (43/01) was donated by Gattefossé, Saint Priest, France. Poloxamer 407 (PX) was obtained from BASF (Germany). Low molecular weight ultrapure chitosan chloride (Protasan UP CL 113) and sodium alginate (Pronova ultrapure alginate MVM, <75 kDa, intermediate viscosity >200 mPa*s) were purchased from (Novamatrix, FMC, Sandvika, Norway). Hydroxyethyl cellulose 250M (HEC) was obtained from SE Tylose GmbH & Co.KG, Wiesbaden, Germany). Alginate lyase, coumarin-6 (cou), Calcofluor-White, MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, mucin from porcine stomach type II, DNA (low molecular weight from salmon sperm), Trizma base, sodium chloride, potassium chloride, 4′,6-diamidino-2-phenylindole (DAPI), and diethylenetriaminepentaacetic acid (DTPA) were purchased from Sigma–Aldrich (Steinheim, Germany). Egg yolk emulsion was obtained from Oxoid Ltd (Hampshire, UK). Bacto Casamino acid was purchased from Difco Laboratories Becton Franklin Lakes, NJ, USA). Alexa Fluor 633 wheat germ agglutinin (WGA) was obtained from Invitrogen (Carlsbad, USA. The live/dead BacLight Bacterial Viability Kit was purchased from Molecular Probes Inc. (Oregon, USA). All other reagents and solvents used were of analytical grade.
Nafee N, Gaber DM, Abouelfetouh A, Alseqely M, Empting M, Schneider M. Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection. Would Nanocarriers Complement Biofilm Disruption or Pave Its Road?. Int J Nanomedicine. 2024;19:3861-3890, https://doi.org/10.2147/IJN.S445955
Read also our introduction article on Alginate here:


