Update: Risk Mitigation of Nitrosamines Formation in Drug Products: Role of Excipients

1. Introduction
The issue of Nitrosamine impurities in drug and excipient manufacturing has become a significant concern for the pharmaceutical industry and health authorities. In June 2018, the FDA was notified of the presence of N-nitrosodimethylamine (NDMA), an impurity found in valsartan, an angiotensin II receptor blocker. In July EMA issued a press-release regarding the recall of some Valsartan medicines following the detection of an impurity. Then, several other medications, including Ranitidine, Nizatidine, and Metformin, have been found to contain unacceptable amounts of Nitrosamines.
The term Nitrosamine describes a class of compounds having the chemical structure of a nitroso group bonded to an amine (R1N(-R2)-N=O). The compounds can be formed by a nitrosation reaction between amines and nitrous acid (nitrite salts under acidic conditions). Secondary amines are of greatest concern. However, tertiary amines can also undergo nitrosation via more complex pathways. All secondary and tertiary aliphatic and aromatic amines should therefore be considered, including those present as part of the starting material, intermediate or final structure as well as those introduced as reagents, catalysts, solvents or as impurities. The reaction can be catalysed by heat and acidic conditions.
Some Nitrosamines are classified as probable or possible human carcinogens by the International Agency for Research on Cancer (IARC). They are referred to as „cohort of concern“ compounds in the ICH guidance for industry M7(R1) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. The guidance recommends that any known mutagenic carcinogen, such as nitroso compounds, should be controlled at or below levels such that there is a negligible human cancer risk associated with exposure to potentially mutagenic impurities.
Several authorities issued guidance (EMA, FDA) and information on Nitrosamine impurities and requested Marketing Authorization Holders (MAHs) to conduct a risk evaluation with regards to Nitrosamine formation in their drug products.
Excipients may contribute to the formation of Nitrosamines through precursor substances present in the excipient (e.g. nitrites, amines). Nitrite impurities are found in most of the commonly used excipients, at least in traces. A recent publication by Boetzel et al. (2022) has reported the nitrite values of commonly used excipients, referencing a database established by Lhasa Limited. However, the original manufacturer names are not disclosed. Furthermore important to note is that the values were measured or provided by different companies within the consortium, and the analytical methods used were not standardized. Therefore, it cannot be ruled out that some of the observed variability may be attributed to the analytical methods employed.
Update
The reason for the update is that MEGGLE invested in the development of a new analytical method to even be able to determine trace levels of Nitrite in Lactose. The new analytical method was successfully validated in cooperation with the Technical University Munich (TUM). Therefore it is an important achievement that with the new method also trace levels of Nitrite can be determined in MEGGLE´s large Lactose portfolio.
2. Lactose: Level of Nitrite and Potential Sources
Lactose has typically a very low nitrite content. In the publication from Boetzel et al. (2022) the nitrite content for Lactose from eight suppliers ranged from 0.07 to 1.7 ppm, with a mean of 0.54 ppm (table 1). Microcrystalline Cellulose showed a similar range from 0.04 to 2.4 ppm, with a mean of 0.70 ppm. Where as Crospovidone had the highest levels with a mean of 8.3 ppm and a maximum value of 14 ppm nitrite.
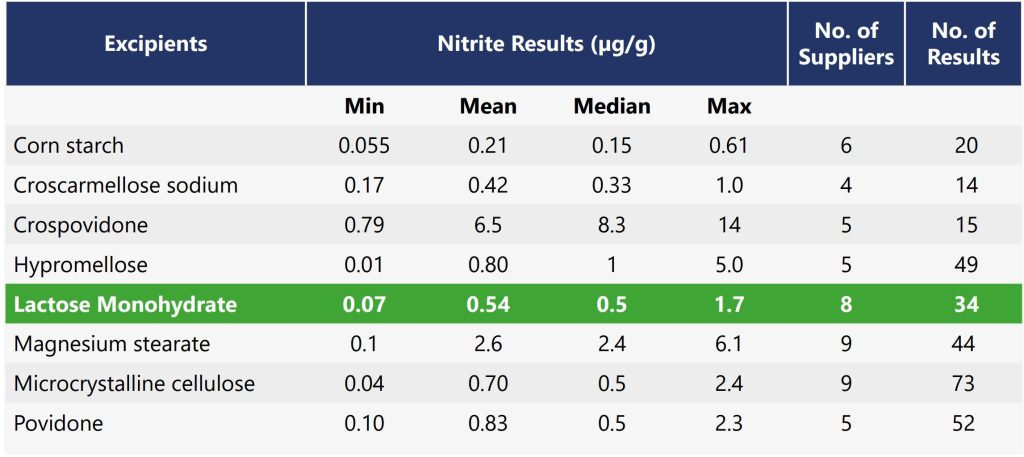
However, as the nitrite contribution is assumed as additive the sum and total amount of all excipients in the formulation have to be considered. As fillers/diluents are typically used in larger proportions they could play an important role even if their nitrite content is comparably low.
There seems to be some confusion in the ongoing discussions regarding the presence of nitrite in excipients (USP Nitrosamine Exchange). On the one hand there is a lot of uncertainty regarding the potential origin of the nitrite. On the other hand, there is a misconception regarding the term „nitrite-free,“ which is not an absolute definition, but rather dependent on the sensitivity of the analytical method or the applied limits. For example, a product can be labelled „non-alcoholic“ in the United States and the European Union if it contains no more than 0.5 % alcohol by volume. The key question is what limits for nitrite are appropriate.
Scientifically derived limits for nitrite levels should be set for critical active ingredients, bearing in mind that „nitrite-free“ excipients may not be achievable. This is simply a matter of method sensitivity (detection limit). Water and other natural materials (plant-based or animal-based) contain at least traces of nitrite.
One of the main sources of nitrate and nitrite is the use of nitrogen-based fertilisers such as ammonium nitrate, urea and ammonium sulfate, which are commonly used to provide crops with the necessary nutrients for growth. Following the biochemical reactions of the nitrogen cycle ammonium is converted to nitrite (NO2-), this is called nitrification. Nitrification is a two-step aerobic biological process that involves the oxidation of ammonium to nitrite, and then further oxidation of nitrite to nitrate (NO3-). Bacteria responsible for these conversions are called nitrifying bacteria and are typically present in soil, water, and wastewater in various species and strains.
Another source of nitrite in water is acid rain. When nitrogen oxides, specifically nitrogen dioxide (NO2) and nitric oxide (NO) are released into the atmosphere as air pollutants. These dissolve in rainwater and form nitric acid (HNO3). Nitric acid can further react with other compounds in the environment and eventually convert to nitrite (NO2-) in water. Nitrogen oxides (NOx) originate from reactions of nitrogen as component of the air with oxygen at high temperatures, typically above 1,000 degrees Celsius, e.g. during combustion.
3. Nitrites in MEGGLE Excipients
We have been monitoring nitrite content of Lactose regularly since many years. As nitrate content is also important for baby food, we have implemented a method, specifically designed for milk products, and that is sensitive enough to determine low nitrite content as required for infant milk products (ISO 14673-3:2007- 05). For our pharma grade Lactose it has been found that nitrite is typically not detectable in the Lactose powder (below detection limit of 0.2 ppm). Notably all values are lower than the mean reported for Lactose by Boetzel et al. (2022). Further information and discussion regarding the analytical method to determine nitrite in excipients is given in chapter 4.
Lactose is a natural material and not chemically synthesized. Organic solvents, catalysts and other reagents which might be a reason for the presence of secondary, tertiary or quaternary amines and nitrite salts are not used. The manufacturing of pharma grade Lactose includes several washing and raffination steps as well as a double crystallization. The manufacturing process does not create the highly acidic conditions necessary for the formation of Nitrosamines and for drying only indirect heating is used. There is no potential NOx generation from combustion.
On the other hand, Lactose monohydrate is isolated and purified from whey which is a by-product of cheese manufacturing. Therefore, nitrite traces might come from the raw material whey and water. Testing on nitrates and nitrites is conducted as part of incoming goods inspection of the whey. The acceptance limits are as follows: Nitrate < 50 ppm, Nitrite < 5 ppm. The process water is regularly monitored according German TrinkwV and showed very low levels below 0.02 ppm over the past three years.
Relevant factors, as well as measured values, can be found in the supplier statement along with the IPEC
Questionnaire for the respective materials.
The IPEC Federation recently provided an updated „Questionnaire for Excipient Nitrosamines Risk Evaluation“ (Feb 2023). This questionnaire reflects considerations for excipients from the guidance from the EMA, including the „Questions and answers for marketing authorization holders“ and the FDA Guidance for Industry „Control of Nitrosamine Impurities in Human Drugs“. The questionnaire includes a matrix to consider the structure and the origin of the excipient as a first indication of risk, as well as measured values for nitrite and Nitrosamines. The use of a standard format facilitates the collection of data from excipient suppliers and assists pharmaceutical manufacturers in their Nitrosamine risk assessment.
It should be noted that the excipient manufacturer cannot carry out a Nitrosamine risk assessment as this requires specific knowledge of the actual drug formulation and the properties of the active substance
Update – New Method & Results
With the new validated, analytical method the determination even of trace levels of Nitrite in Lactose is possible. The new method is based Ion chromatography (IC), this is a liquid-solid chromatographic method used to separate and determine ionic solutes. For IC-Analytics different columns as solid phase and different eluent systems as liquid phase can be selected. The exchange and separation of ions depends on their charge and affinity towards the applied stationary phase (ion exchange column). The separated ions are then detected and quantified using conductivity measurement or UV/VIS spectroscopy. The most common used columns are hydroxide-selective anion-exchange columns, carbonate eluent anion-exchange columns, cation-exchange columns, ion-exclusion columns, amino acid columns and reversed-phase columns. The IC method set-up has been optimized with regards to columns, eluent system, gradient and flow rate as well as the sample preparation to achieve a good resolution for the quantification of Nitrite in the lactose matrix. Based on the method validation with lactose the Limit of Quantification (LOQ) is 0.03 ppm and the Limit of Detection (LOD) is 0.01 ppm.
After the successful validation of the new method, at least 3 samples from different product groups (sieved, milled, agglomerated, spray dried and anhydrous lactose, as well as milled and sieved inhalation grade lactose) have been analyzed. All results showed a value below 0.01 ppm (table 2).

For MEGGLEs pharma grade Lactose portfolio this means that with Nitrite values below 0.01 ppm, they are not detectable, so basically “nitrite free”. This lowest amount of nitrite also in MEGGLEs direct compressible (DC) lactose grades (agglomerated, spray-dried and anhydrous lactose) makes them a perfect solution to mitigate/reduce risk of nitrosamines formation for drug product manufacturing.
4. Strategies for Mitigating Risks in Drug Product Manufacturing
As first step the risk of Nitrosamine formation or impurities in APIs has to be assessed. The recent publication from Nanda et al. “Inhibition of N-Nitrosamine Formation in Drug Products investigating possible APIs and formation mechanisms” (2021) provides valuable insights.
The EMA requires Marketing Authorization Holders (MHA) to perform the following risk assessment steps:
- Step 1: MAHs to perform a risk evaluation to identify if APIs and/or Finished Products could be at risk of presence of Nitrosamine
- Step 2: If a risk is identified: MAHs has to proceed with confirmatory testing in order to confirm or refute the presence of Nitrosamines.
- Step 3: If the presence of Nitrosamine(s) is confirmed:, MAHs should implement effective risk mitigating measures through submission of variation.
When a (potential) risk of Nitrosamine formation has been identified, the best risk mitigation strategies have to be evaluated. Besides the API manufacturing process and possible adaptions thereof, the formulation and used excipients as well as the drug product manufacturing process should be reviewed and if needed variations filed. A detailed description and points to consider can be found in the Q&A from EMA, in the following paragraph we highlight only aspects regarding excipients and drug product manufacturing process.
See the full technical brochure on “Update Nitrosamines” here
(click the picture to download the brochure)
Source: MEGGLE technical brochure “Update Nitrosamines”
Do you need more information or a sample of MEGGLE excipients?


