Skin Penetration and Permeation Properties of Transcutol® in Complex Formulations
Abstract
Percutaneous delivery is explored as alternative pathway for addressing the drawbacks associated with the oral administration of otherwise efficacious drugs. Short of breaching the skin by physical means, the preference goes to formulation strategies that augment passive diffusion across the skin. One such strategy lies in the use of skin penetration and permeation enhancers notably of hydroxylated solvents like propylene glycol (PG), ethanol (EtOH), and diethylene glycol monoethyl ether (Transcutol®, TRC). In a previous publication, we focused on the role of Transcutol®as enhancer in neat or diluted systems. Herein, we explore its’ role in complex formulation systems, including patches, emulsions, vesicles, solid lipid nanoparticles, and micro or nanoemulsions. This review discusses enhancement mechanisms associated with hydroalcoholic solvents in general and TRC in particular, as manifested in multi-component formulation settings alongside other solvents and enhancers. The principles that govern skin penetration and permeation, notably the importance of drug diffusion due to solubilization and thermodynamic activity in the vehicle (formulation), drug solubilization and partitioning in the stratum corneum (SC), and/or solvent drag across the skin into deeper tissue for systemic absorption are discussed. Emphasized also are the interplay between the drug properties, the skin barrier function and the formulation parameters that are key to successful (trans)dermal delivery.
Introduction
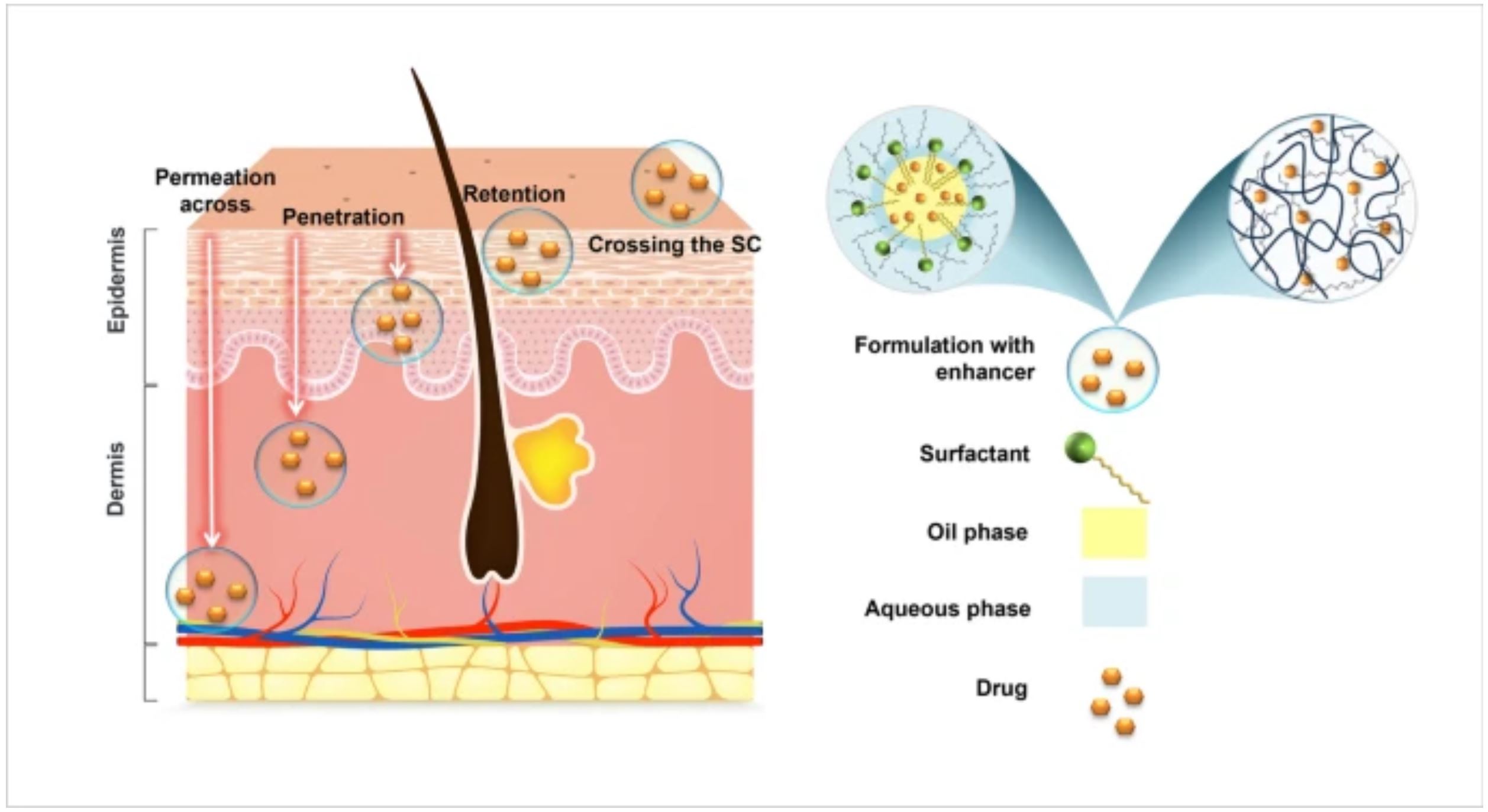
Enhancement of drug delivery to/via the skin has been guided by universally recognized strategies which include i) maximizing the thermodynamic activity of the drug in its vehicle; ii) raising the solubility of the drug in the stratum corneum (for local delivery); and/or iii) enhancing drug diffusivity across the stratum corneum for transdermal delivery [1,2,3]. These seemingly simple guidelines envelope complexities that arise from concomitant interactions of the formulation components with the skin structures. Maximization of thermodynamic activity in itself is a balancing act of achieving the right degree of drug solubilization relative to drug’s saturation solubility in the vehicle. Moreover, the drug and the formulation constituents (solvents, cosolvents, emulsifiers, oils) including water can interact with the skin to different degrees and rates in synergistic or competitive manner. Meanwhile, “the most innocuous of vehicles may change the nature of the stratum corneum” when hydration occurs in the membrane [2, 4]. Thus, understanding the mechanism by which an excipient exerts its function vis-à-vis the formulation and the skin can be critical to the development process.
Percutaneous absorption is governed by the physical chemical properties of the drug and the physiology of the healthy or diseased skin. A non-invasive approach to these immovables lies in formulation optimization, including the incorporation of solubilizers and one or more penetration / permeation enhancers. Hundreds of chemical entities have been described as enhancers for having shown some type of penetration / permeation enhancing effect – irrespective of the mechanisms in play. However, not all of the revealed chemical entities have pharmaceutical acceptance due to concerns over their safety / innocuity. As for enhancers with precedence of use, published reviews provide useful information on their properties which is largely based on simple, neat, or diluted experimental models. In fact, there are fewer reviews discussing their role in complex formulation systems where they may exert multiple effects [1, 5, 6]. Meanwhile, the commonly used excipients (oils, surfactants, cosolvents) and water are known to exert one or another effect on drug penetration and permeation and therefore behave as “enhancers”. The interplay between the formulation constituents and the resulting effect on penetration and permeation is yet another confounding factor to consider.
The aim of this review is to explore hydroalcoholic solvents in general and the function of Transcutol® (TRC) in particular for their effects in a variety of formulation systems, in multi-component settings alongside other solvents and enhancers. Following an overview of enhancer categories and the basic concepts about skin penetration and permeation enhancement, the effects of TRC and other hydroalcoholic solvents are discussed in the context of gels, emulsions, ointments, patches, as well as complex formulation systems like vesicles, solid lipid nanoparticles, and micro and nanoemulsions. Where possible, the factors influencing the outcome of the studies, relative to therapy objectives, and the possible interplay among the constituents are highlighted.
Download the full article as PDF here Skin Penetration and Permeation Properties of Transcutol® in Complex Formulations
or read it here
About Transcutol
The tradename Transcutol® refers to highly purified (≥ 99%) cosmetic grade (CG), veterinary injectable grade (V), and the pharmaceutical P and HP grades of DEGEE. Transcutol® P and Transcutol® HP, referred to simply as TRC in this manuscript, have guaranteed purities of ≥ 99.7% and ≥ 99.9% respectively [21]. The elimination of impurities is critical to the safety of the product. For example, impurities like ethylene glycol and diethylene glycol may have contributed to cell culture viability outcomes in a 2019 publication [33] where a reagent grade of DEGEE (Millipore sigma) was used.
TRC is globally approved for topical and transdermal use (30, 31) at concentrations of up to 49.9%. Marketed dermal products in Europe have not produced contra-indication/adverse effect linked to pregnant women. There are topical references for adolescents (US/Canada) and orally administered products for adult and children under 6 years of age in Europe. Full safety / tox data on TRC is available from the manufacturer, the FDA drug master file, and published works [34,35,36,37,38] indicating it can be administered safely to humans. The proposed permissible daily exposure limits, with 100-times safety factored into the calculations are 20 mg/Kg/day for dermal and 10 mg/Kg/day for oral administration, translating respectively to 1400 and 700 mg/day for a 70 kg man. The FDA Inactive Ingredients Database cites a significantly higher daily exposure limit of 1720 mg/day in a topical product. TRC is currently referenced in veterinary and human injectables outside the USA. The suitability of TRC in parenteral applications is in evidence by a study that quantitatively analyzed the effect of eighteen different excipients on human hemoglobin, including eleven which are approved for parenteral use by the FDA [35]. The results pointed to the non-hemolytic nature of TRC in the ranks of medium chain triglycerides (Labrafac® WL 1349).
Musakhanian, J., Osborne, D.W. & Rodier, JD. Skin Penetration and Permeation Properties of Transcutol® in Complex Formulations. AAPS PharmSciTech 25, 201 (2024). https://doi.org/10.1208/s12249-024-02886-8
Read also our introduction article on Topical Excipients here:


