New Drug Formulations and Their Respective Generic Entry Dates
BACKGROUND: After new prescription drugs reach the market, manufacturers
sometimes create modified versions of them. These new formulations
can expand patient treatment options, but they may also be protected by
later-expiring patents or data exclusivities, which can lead to later generic
entry for the new formulations compared with the original product.
OBJECTIVE: To quantify how frequently manufacturers introduce new
formulations of existing drugs and how often these new formulations earn
additional years of market exclusivity beyond that of the original product.
METHODS: Using a cohort design and FDA databases, we assessed how
frequently manufacturers introduced new formulations of 17 new smallmolecule
drugs approved in 2002 and when generic entry for the new formulations
and original product occurred.
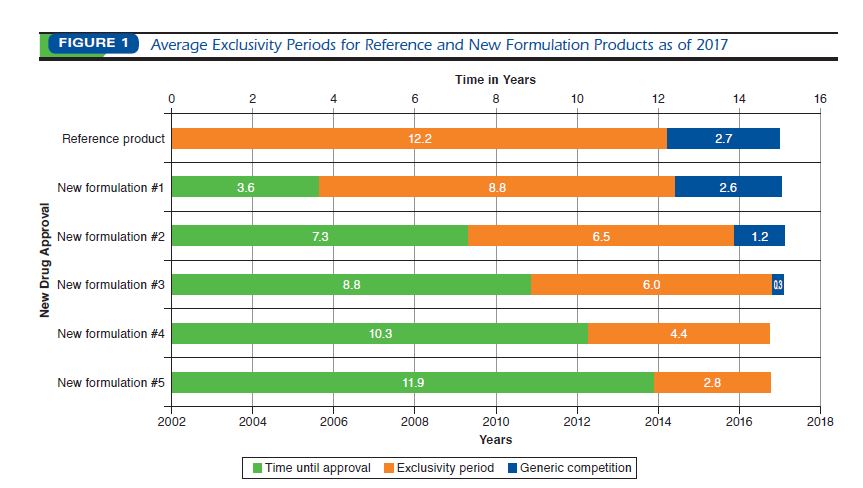
RESULTS: Through 2017, nine (53%) drugs approved in 2002 had been connected
to 21 new formulations, most (11/21, 53%) introduced before 2007.
Generic entry was observed in 6 of 9 (67%) cases and occurred more than
2 years later for the new formulations in 3 of the cases.
CONCLUSIONS: Our results suggest that the introduction of new formulations
of brand-name drugs occurs in about half of cases and sometimes
provides manufacturers with a lengthy period of additional market exclusivity
beyond that of the original product.
Interested in the full pdf New Drug Formulations and Their Respective Generic Entry Dates
Reed F. Beall, PhD; Aaron S. Kesselheim, MD, JD, MPH; and Ameet Sarpatwari, JD, PhD
J Manag Care Spec Pharm. 2019;25(2):218-24

