Factors affecting the stability and performance of amorphous solid dispersions of poorly soluble active pharmaceutical ingredients
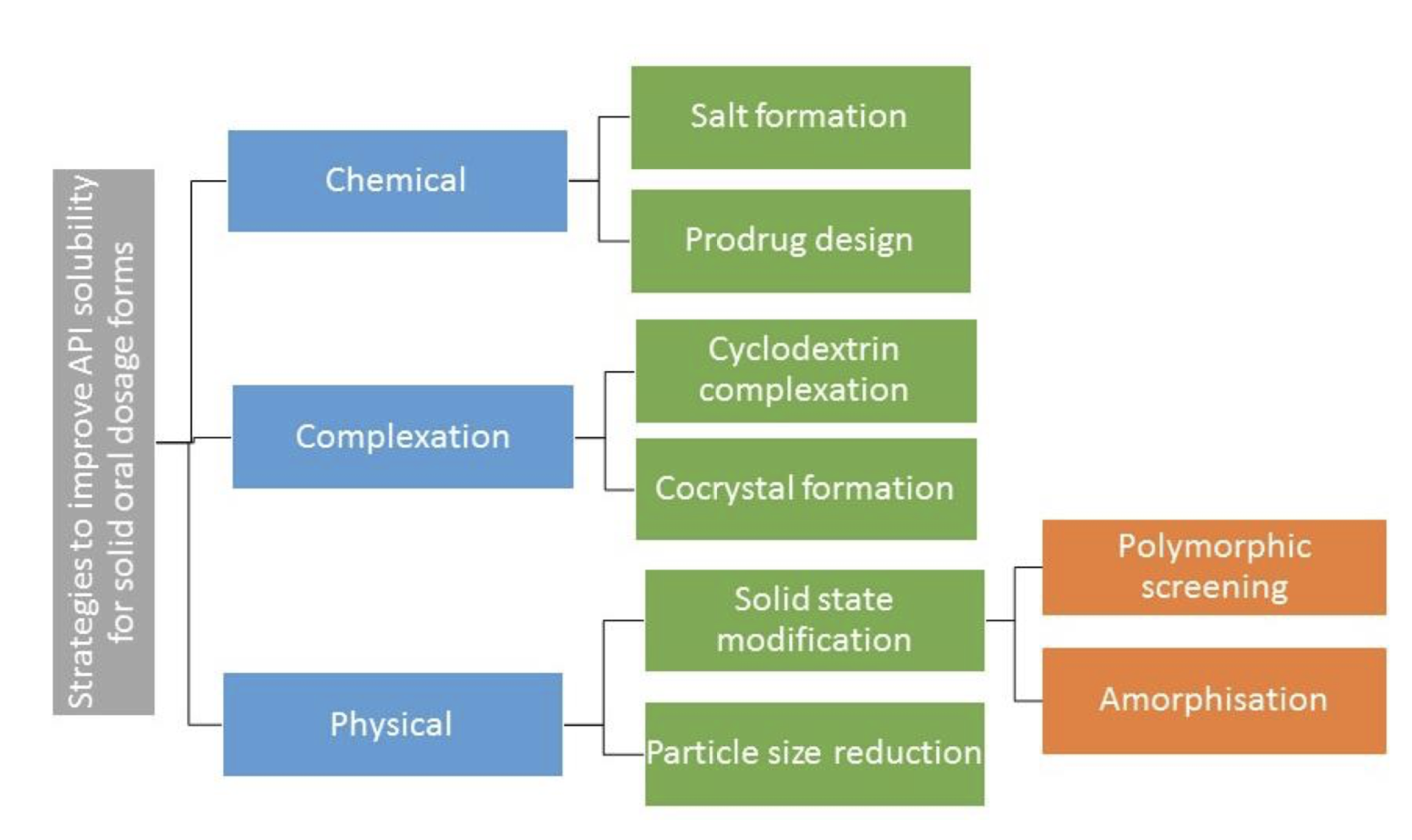
Over the last twenty years, the poor solubility profile of pipeline drugs has limited their development as solid oral dosage forms. Formulating these APIs as amorphous solid dispersions is one strategy to overcome their poor aqueous solubility. The major limitation to amorphous solid dispersion development is the physical instability of the amorphous state. The objective of this thesis is to investigate factors affecting the physical and chemical stability of amorphous solid dispersions.
The work carried out in this thesis has demonstrated that the chirality of an API is a factor which should be considered when developing an amorphous solid dispersion, particularly when cellulose- based polymers are used, as chiral recognition may exist between the polymer and the API. This was demonstrated to be true for opposing enantiomers of ibuprofen and the cellulose polymer, HPMC, when cryo-milled together. The S-ibuprofen-HPMC system contained significantly less crystalline ibuprofen than the equivalent R-ibuprofen-HPMC system. However, this stereoselective effect was diminished when the amorphous solid dispersions were produced via spray drying. This is believed to be due to the superior molecular mixing facilitated by solubilising both components prior to spray drying, which enabled a greater extent of amorphisation for both enantiomeric systems. It was also discovered that when the R,S-ibuprofen is formulated as an amorphous solid dispersion with HPMC, a racemic switch (to the S enantiomer) can increase the amorphous ibuprofen content and increase ibuprofen’s dissolution rate.
The choice of polymer in an amorphous solid dispersion formulation is acknowledged to influence amorphous solid dispersion stability and performance. However, a comprehensive evaluation of the effect that polymer physicochemical properties may have on amorphous solid dispersion stability and performance, which would aid polymer selection at an early stage of development, is lacking. The relationship between several physicochemical properties of poly-vinyl polymers, such as molecular weight and co-polymer substitution ratio, and ketoprofen amorphous solid dispersion stability and performance was determined. The relative humidity induced glass transition value was found to be useful to describe the effect that co-polymer substitution ratio has on moisture sorption and associated plasticisation. It was also discovered that it is the aqueous solubility of the polymer, rather than the complete amorphisation of ketoprofen in the dispersion, which is the critical factor determining the degree of ketoprofen supersaturation which is achievable.
The relationship between the route of amorphous solid dispersion generation and performance was also evaluated for two processes; electrospraying and spray drying. It was determined that solution conductivity had no impact on the morphology of the particles produced via spray drying, while solutions with lower conductivity, when processed via electrospraying, produced particles which displayed webbing. The drug loading, physical state and dissolution profile of the ketoprofen- PVP material was similar for both electrosprayed and spray dried material. However, the smaller particle size associated with the electrosprayed material resulted in poorer compressibility and higher surface moisture sorption rates compared to the equivalent spray dried material.
Factors affecting the chemical stability, specifically the photostability of spray dried amorphous solid dispersions of nifedipine were also investigated. It was discovered that the solvent composition of the solution which was spray dried was critical to the photostability of the amorphous solid dispersion, which is believed to be due to its effect on the surface enrichment of nifedipine.
Lastly, the potential of amorphous solid dispersions of nifedipine to be used in the treatment of autonomic dysreflexia, a hypertensive crisis, was explored. Soluplus and HPMC were determined to be unsuitable polymers for this purpose due to poor nifedipine release profiles attained from the solid dispersions produced, while PVP-based systems exhibited nifedipine release profiles which were similar to the nifedipine release profile obtained from a ruptured Adalat® capsule, which is the current standard of care. Clearly, the PVP-nifedipine amorphous solid dispersion formulations warrant further investigation for the treatment of this condition, as they may represent an improvement on the status quo. Download the full thesis here
Thesis by Emer Browne – School of Pharmacy and Pharmaceutical Sciences Trinity College Dublin, The University of Dublin – Factors affecting the stability and performance of amorphous solid dispersions of poorly soluble active pharmaceutical ingredients

