Water-assisted synthesis of mesoporous calcium carbonate with a controlled specific surface area and its potential to ferulic acid release
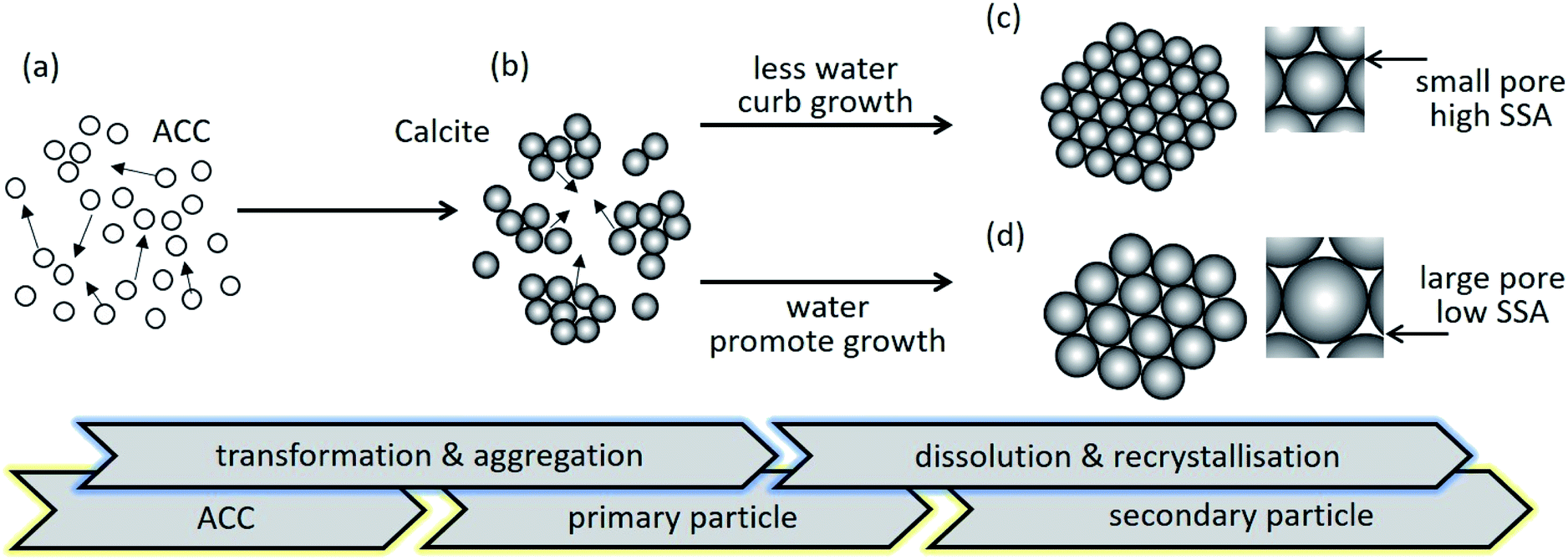
A carbonation process to control the specific surface area of mesoporous calcium carbonate and the dissolution profile of ferulic acid on mesoporous carbonate particles are presented. The effects of water content on the physicochemical properties, specific surface area, pore size, crystallinity, and morphology are evaluated. Mesoporous calcium carbonate particles are synthesised with well-controlled specific surface areas of 38.8 to 234 m2 g−1. Each of the submicron-size secondary particles consists of a primary particle of nano-size. During secondary particle formation, primary particle growth is curbed in the case with less water content. By contrast, growth is promoted via dissolution and recrystallisation in the presence of water. The release rates of ferulic acid are gradually enhanced with increasing specific surface area of the mesoporous calcium carbonate, that reflects crystallinity of ferulic acid.
Conclusions
We proposed a carbonation process to synthesise the calcite phase of mesoporous calcium carbonate with a well-controlled specific surface area (38.8–234 m2 g−1). The water content in the precursor of ACC dispersion played a crucial role on pore formation. Then we demonstrated the release tests of FA on mesoporous calcium carbonate. The mesoporous calcium carbonate with the highest specific surface area of 234 m2 g−1 had the highest release of FA. The fluorescence spectroscopy study showed the model fluorescence compound was dispersed in the molecular state when using a higher specific surface area of mesoporous calcium carbonate. This result reflected the release behaviour of FA. Access the full publication here

