3D printing fabrication of Ethylene-Vinyl Acetate (EVA) based intravaginal rings for antifungal therapy
Vulvovaginal candidiasis is a vaginal infection that affects women of reproductive age. Nowadays, the high administration frequency of conventional antifungal formulations, and recurrences negatively impact patients’ well-being. In this context, intravaginal rings (IVRs) offer the possibility of controlled local drug delivery with one single application, thus possibly increasing patient compliance.
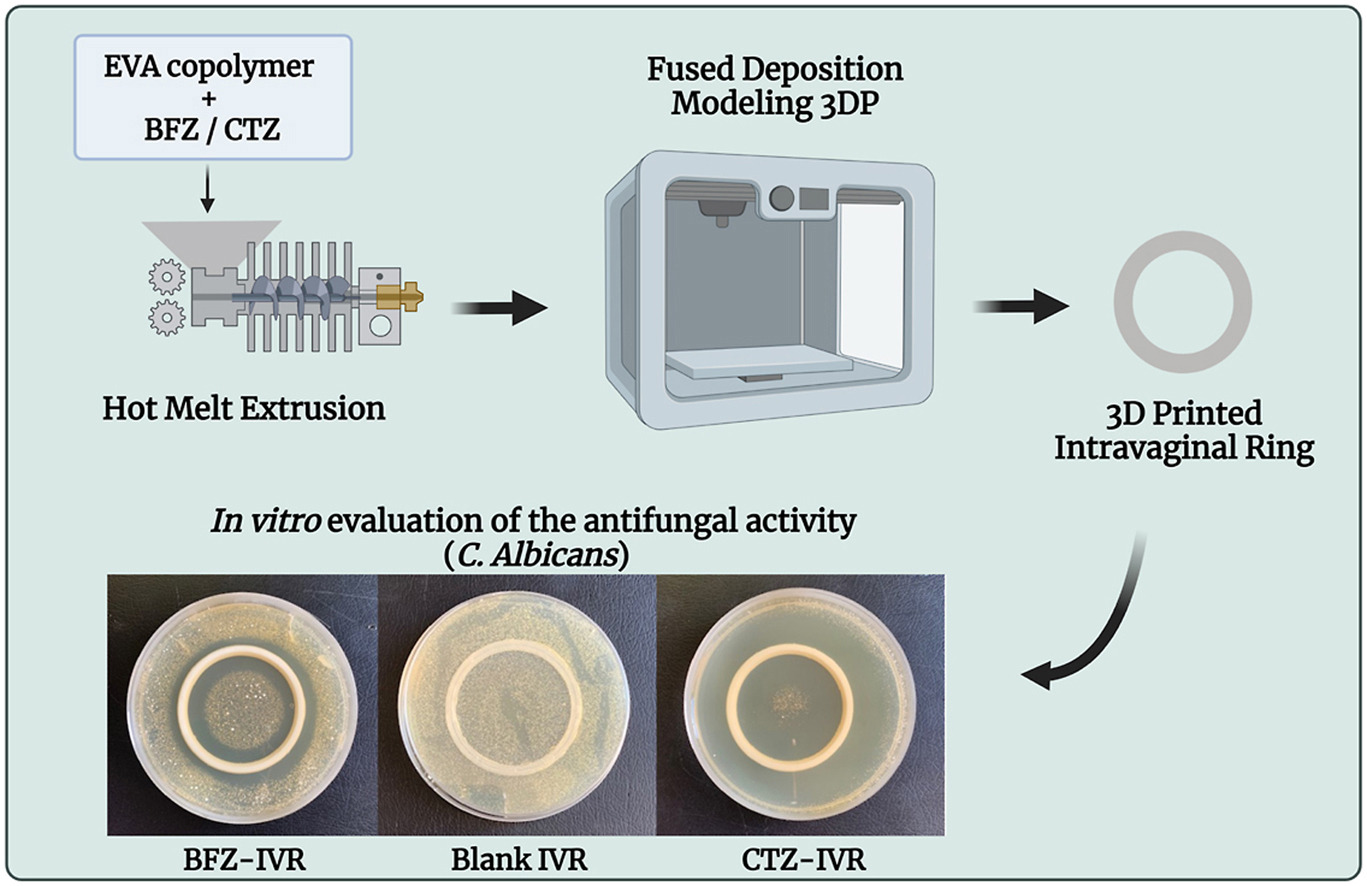
This project aimed to fabricate 3D printed IVRs to highlight the potential application of these medical devices for antifungal therapy, as well as emphasize the employment of 3DP as alternative manufacturing tool. Ethylene-Vinyl Acetate copolymer was chosen as matrix, and the antifungal efficacy of bifonazole and clotrimazole loaded in the IVRs was compared. The resulting medical devices were characterized using Fourier Transformed Infrared spectroscopy, the thermal behavior was investigated with Thermogravimetric Analysis and Differential Scanning Calorimetry, proving the stability of the incorporated drugs.
In addition, the drug release profile was evaluated in a vaginal fluid simulant pH 4.2, at 37 °C, showing a sustained release over a week. The compressive strength of the IVRs was investigated, confirming that the mechanical properties comply with the already commercialized devices. To evaluate the antifungal activity, an in vitro time-kill assay was performed against Candida albicans for 7 days, exhibiting a complete growth inhibition after 4 days for the 3D printed IVRs. Overall, this work represents a step forward in the production of 3D printed IVRs potentially able to exert antifungal activity with one single application.
Introduction
Vulvovaginal candidiasis (VVC) is a frequent and common infection of the vulva and/or vagina predominantly caused by the pathogen fungus Candida albicans, or related fungi. After bacterial vaginosis, it is the second most common cause of vaginal infections, affecting millions of women every year [1]. C. albicans is commonly found on the mucous surfaces of the human body, being part of the quiescent flora. However, changes in the environment can promote proliferation and infection development, characterized by the following symptoms including itching, vaginal soreness, irritation, burning, swelling, dyspareunia, external dysuria, and abnormal vaginal discharge [2,3]. The pathogenesis depends on the virulence of the Candida and on the defence mechanisms of the individual. Some predisposing factors and triggering mechanisms have been identified, such as the use of antibiotics, uncontrolled diabetes, use of contraceptives, and hormonal genetic and lifestyle-related factors [[4], [5], [6]].
The conventional treatment of candidiasis consists in administering antifungal drugs, available in a variety of standard formulations, such as pessaries or creams, for a duration from three to seven days [7]. Among antifungal drugs, the class of imidazoles is still considered the first-line treatment for C. albicans infections. This class of drugs inhibits the enzyme lanosterol 14-alpha-demethylase, cytochrome P450 dependent, which is essential for sterols biosynthesis. Moreover, given the high incidence of recurrences, the maintenance regimen is recommended including oral weekly antifungal drugs for up to 6 months [8]. Despite the therapeutic advances, the high frequency of treatment administration, the onset of symptoms, microbial resistance, and recurrences still impact the psychological well-being of patients, resulting in poor compliance [9,10]. Thus, finding an efficient strategy is needed. Recently, topical solid vaginal formulations have aroused great interest, including IVRs [11]. IVRs are flexible medical devices that provide a continuous and sustained, or controlled local delivery of the drug incorporated, with a single application [12]. Commonly, they are manufactured by hot melt extrusion (HME) or injection moulding (IM), using silicone elastomer and polyurethane [13]. Based on the advantages conferred, such as the safety and the low side effects, IVRs are well-accepted by women [14] being already established for hormonal therapy [15]. Additionally, their feasibility and efficacy in other applications associated with women’s health have been investigated (e.g., for the overactive bladder treatment [16], for the prevention of sexually transmitted diseases [17], and as cervical ripening agents [18]). However, at present, their application for antifungal treatment has been poorly explored.
Our group has previously demonstrated the possibility to produce antifungal IVRs by 3D printing using thermoplastic polyurethane [19].
The employment of 3DP rather than IM, as manufacturing tool, enables the production of cost-effective small batches, centred on the patient’s need or useful for clinical studies. Complex geometries can be produced, and the size and doses can be easily customized thanks to 3DP that allows rapid prototyping [20,21]. Recently, few applications of 3DP in the production of intravaginal devices have been reported [[22], [23], [24]].
This project aimed to make a step forward on the development of a new generation of 3D printed IVRs, for the treatment of recurrent fungal infections. For this purpose, ethylene-Vinyl Acetate (EVA) copolymer was chosen as matrix and the loading and efficacy of bifonazole (BFZ) and clotrimazole (CTZ) as antifungal imidazole drugs was compared.
EVA is a biocompatible, insoluble, and non-toxic thermoplastic copolymer of ethylene and vinyl acetate, which has FDA approval, and it is a plastic material suitable to produce particularly elastic products, which stand out for their softness and flexibility [25,26]. In recent years, thermoplastic EVA copolymers have shown great potential for the manufacturing of sustained-release matrices. An example of an EVA-based device, that is available on the market, is the contraceptive IVR Nuvaring®, where the active pharmaceutical ingredients (API) (etonogestrel and ethinylestradiol) are homogeneously distributed within the core polymer [27].
Herein, IVRs were 3D printed with Fused Deposition Modeling (FDM) technique, coupled with HME to produce the filaments needed for the printing process. FDM is commonly use in the pharmaceutical field, being versatile, low cost and given the wide range of material available [28]. After an initial characterization comprising size, weight, and drug content homogeneity, the chemical composition, thermal studies and mechanical properties were evaluated. Furthermore, the release profile was investigated using a mixture of water/ethanol, for quality control intent and a vaginal fluid simulant (VFS) at pH 4.2 to better mimic the in vivo environment. Finally, to assess the antifungal potentiality of the devices, the in vitro antifungal activity was investigated against C albicans for up to 7 days.
Read more
Sofia Moroni, Francesca Bischi, Annalisa Aluigi, Raffaella Campana, Mattia Tiboni, Luca Casettari, 3D printing fabrication of Ethylene-Vinyl Acetate (EVA) based intravaginal rings for antifungal therapy, Journal of Drug Delivery Science and Technology, Volume 84, 2023, 104469, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2023.104469.
Read more on Overview of Pharmaceutical 3D printing here:


