On-demand webinar: 3D printing of tablets – A new era of pharmaceutical manufacturing
Additive manufacturing is gaining increasing attention in the field of pharmaceutical manufacturing. Evaluations are mainly driven by advances in individualized therapy and fast track development of clinical trial material. Melt based systems provide the additional advantage of enhancing the solubility of low soluble compounds by creating an amorphous solid dispersion, where the amorphous form of the drug substance is stabilized within a polymer matrix.
In this webinar from ISP India, Dr. Thomas Kipping from Merck is presenting a novel melt drop technology where the 3D printed geometry is created by individual deposition of polymer droplets.
Find out more about Merck’s solutions for 3D printing here!
Transcript
Speaker 1: Sharing knowledge is a passion with ISP India, as we believe that knowledge is more powerful when it is shared. Good afternoon. I’m Modi Shetty, and as always, it is a pleasure to welcome you all to another session of pharma best practices webinars. We are very pleased to host today’s session by Dr Thomas on use of 3D printing technology in pharmaceutical manufacturing. This is the 90th session on this platform. Since the platform was launched in March 2020, we are so pleased that only 5000 professionals have attended our boss sessions life and another 60000 or so I have watched recordings of these webinars on our YouTube channel. We thank you for taking your time out for these webinars and for your trust and faith in us. This is what keeps us going. Please do visit our website specifically designed for webinars. pbpw.in. For information about webinars planned in the next three months, we have some fantastic webinars planned, so please do visit the website. Let me begin today’s proceedings with a special announcement for all those who are not ISP members. We are having an attractive offer to join ISP, for just 84 US dollars. You can get a two year membership of ISP. Do not miss this offer. Please visit our website pbpw.in the link for which is given in the chat. In today’s webinar. Dr. Thomas will present a novel multidrug technology where the 3D printed geometry is created by individual deposition of polymer droplets. Dr. Thomas is head of drug carriers that book with the broad expertise in the field of solid dosage forms. Thomas provides a deep understanding of formulation development and process optimization in the complex area of pharmaceutical manufacturing. He has a strong background in pharmaceutical industry, including industrial development, GMP manufacturing, clinical supply, research and development. Thomas is a pharmacist by education and holds a Ph.D. in pharmaceutical technology. Presentation by Dr. Thomas will be fought about 50 to 60 Minutes. There’ll be plenty of time for Q&A at the end of the presentation. Please use the question Stop to type your questions. Ladies and gentlemen, without further ado, let me hand over this what your platform to Dr. Thomas. Over to you, Thomas.
Material: Parteck® MXP
Speaker 2: Thank you, Bill. Thank you for that kind introduction. Yeah, and let’s start with today’s talk. 3-D printing of tablets. A new area of pharmaceutical manufacturing. Today, we will have a short introduction here about additive manufacturing for pharmaceutical applications. Then I will show you the advanced melt drop deposition. What is this system? What is the technology behind? Then we will also see how can we combine additive manufacturing with solubility enhancement, especially looking into the 3D printing of amorphous, solid dosage forms. Then we will discuss the future potential, especially focusing here on melt based 3D printing technologies. Yeah, let’s start with a short introduction here. Let’s look at the overview of 3D printing technologies in the pharmaceutical industry. You know, 3D printing is a very broad area. There’s not only one technology, we see a lot of different technology platforms here forming. And they are slightly differing on one hand on the system they’re using on the technology, but also then on how you create this dosage forms. So also, which energies or which additional excipients would you need here in order to create the forms?
Let’s start on the left side. We can see some powder based system where you create your 3D printed dosage forms out of the powder bed. There is also different technologies on the left side. Here you can see the drug on powder or so-called binder jetting here, or you have a liquid binder and then you use this binder to agglomerate each layer. On the other hand, you can use the selective laser sintering. This is also something you can see here on the right side, where you use an energy of a laser beam in order to melt slightly the surfaces and then create your 3D structure out of the powder bed. Another technology you can utilize here. It’s a liquid based system here on the right side. There’s a drop and drop deposition or even the stereolithography. There’s also a technology you can use energy, for example, by UV laser or even by temperature. But usually this kind of technologies they need, it’s kind of a starter in order to really induce a polymerization that is sometimes critical, especially when it comes to unstable drug substances. Another very interesting technology to focus on today are the extrusion based systems. You can see here most known technology, for example, is the FDM technology, the fused deposition modelling technology or even a semi-solid form created by pressure assistance syringes.
Usually you are just using the temperature here in order to generate the homogeneous melt that you can then use to deposit the individual straints. What are the future potential applications of 3-D printing? That is an important question that we need to think of because we will see here with the future we expect, for example, the diagnostic tools to increase. So in the future, you can see we get more data of the patients who get more ideas on what is their metabolism about how, what, how is their metabolism adapting to the individualized personalization to the medications? And then this will be in the future also linked with digital prescription tools. So that goes well, together with the idea of really designing individual tablets for individual patients, so that could lead to the design of the tablet that is then translated in the 3D printed form, and that leads us to the personalized medications. The important point here is not only a variation of those, but also really focusing on the targeted therapeutic effect. So we cannot only operate a dose here that would be also possible with other personalized systems. But here the advantage would be we can also adapt the release kinetic or even we’re going to combine different drug substances. Another challenge that we see, especially in pharmaceutical development, is the change of solubility. We can see here the BSC class system on the left side here you can see the BCS class system.
Think you’re very familiar with this kind of system in order to classify drug substances, according to the solubility, but also that permeability. And if we now look here at the center, you can see the current distribution of marketed substances and you can see we have a high amount of BSC class one and BCS class two, but the BCS one right now is very high. Thirty five percent is as close to around 30 that are the most important ones to look into. And you can see the picture if you look at the drug substances in the pipeline. On the right side, you can see the amount of substances that are currently in the pipeline in development, and you can see there is a big shift coming up. So the BCS class one will be drastic, you’re drastically reduced. Only five to 10 percent are expected. And the majority here will move around BCS class two compounds, but on 60 to 70 percent, we’ll be having challenges with solubility. So for us, it’s an important driver also here to look into the 3D printing technology and to combine the 3D printing together with the bioavailability enhancement.
It is an important an important breakthrough here to fully utilize this potential. We look at solid dispersions, and it’s also interesting to see how where the solid dispersion evolving. Both generations rather based on crystalline carriers like urea and sugars. The second generation are rather polymer carriers and they are usually the well-known polymers that you know, when the hot-melt extrusion process. And the third generation that is actually what is happening right now here we see a mixture of surfactants and polymers. Surfactants itself and surface active polymers already or even mixture of polymers. There’s also a lot of movement here looking into tannery systems in order to create this self-emulsifying surfactant activity. Fourth generation, it’s a sudden controlled release to this dispersions, yet they can be tackled by release modifying polymers, and that is even moving into the new advantages here of 3D printing. And that’s also where we see a great advantage of polyvinyl alcohol as a basic polymer because we can really combine here this strategy here of having a very stable polymer, but then also having a surface activity when it comes to tailoring the release and then keeping even low soluble compounds longer in solution.
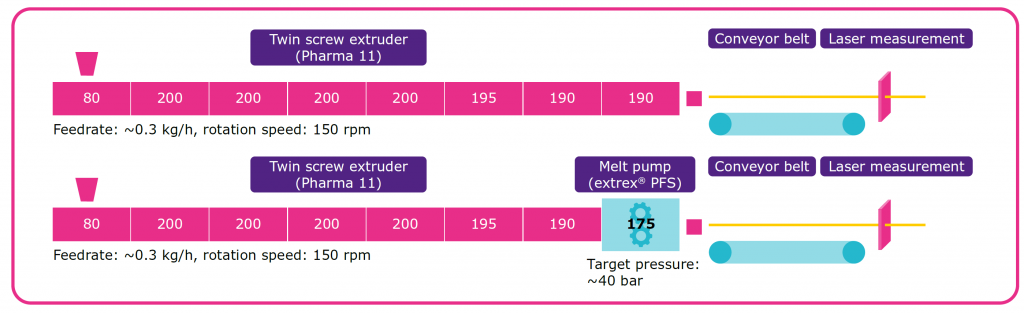
That’s why we created an Parteck MXP here that was initially developed for hot-melt extrusion, its use of the PVA polyvinyl alcohol hydrolisis degree between 85 and 89 percent. And then we have a molecular mass of about thirty two thousand. And on the left side you can see the product properties to really focus on having a good flowability in order to be able to have a homogeneous feeding in an hot-melt extrusion system. That is a very important point, and we have a very good reliability of the process and can have a very reliable, reproducible results here when we create different batches. It’s very important. Another important point is the thermal behaviour, you can see the Tg, the Tm. So the melting point of the polymer and the degradation, and we can see we have a very broad processing window here with the Parteck MXP. And an important point, if you look at this graph, you can see the viscosity plotted against the shear rate. So what you can see is that with an increased shear rate, it was positive, slightly dropping and that we always hear the processing, especially if you think about you have your hot-melt extrusion process itself and then you have a downstream get through very tiny nozzles or a. Then this will help you in order to process your material in this material. So the viscosity will drop and make it easier here in the downstream. Shortly talking about hot-melt extrusion. That is an important technology that we also want to utilize for this new opportunity for this new additive manufacturing. And basically here it’s a process with the twin-screw extruder here from the standard former procedures, which involves the heating, mixing and melting of an API together with a polymer to see the schematics you hear of the process.
API polymer here, premixed already in the feeder, but you can also separately do that later, so here is an example of where we pre-mixed before and then after melting, mixing in here with this certain mixing screw element or needing blocks. And then we have this homogeneous dispersion that we can use for downstream and the advantages of this process for sure that it is a continuous process. We can run it solvent free and there is already a very strong process understanding, especially coming from plastics industry. The process already very well known, very well adapted, so it can easily transfer here into pharmaceutical industry and it’s already there. So that is an important technology for having a continuous process. Yeah, coming from there, we also want to see how can we use the concept here by additive manufacturing?
And now I really want to introduce to you the advanced melt-drop deposition. This kind of new technology that is initially developed here for the plastics industry. And we can see here the system, itself, how it works based on the above plastic reforming. And now you can see here on the right side, you can see the feeder, the feeding unit. So what have you put in there and your drug substance already pre-mixed and dose it here into the barrel inside the barrel there is a single screw. So it’s the single screw extrusion system that is feeding the premix material here through the different temperature zones, and you can see the three different temperature zones. And while the material here is passing, the screw, screw is rotating and feeding the material forward here into the gap here to the reservoir. Then the material here is getting melted. We create a polymer melt. And then this polymer melt here fills up the reservoir at the end where you can see the screw tip. And know something very important happens because of this technology. We don’t generate the pressure by the rotation of the screw. We can generate the pressure by translational movement of the screw itself.
On the right side, here we apply a force that pushes together here on the reservoir. That’s why we can create very high pressures and go up to 500 bar or even higher. Now, the important part of the technology is really the outlet, if you look here on the left side, you can see in yellow, that is here. Yeah, the needle, the needle in yellow. And you can see below there is the channel of the reservoir, and this needle here opens and closes moves up and down here at a very high frequency because here on the top here there is a piezo actuator and the two actuator here can be run at a very high frequency. So we can really have the piezo actuator running at about 150 yards, that means 250 times per second. It can open and close. And each time it opens and closes, we can deposit one individual droplet and thereby we can create our three dimensional structure by depositing individual droplets. So very nice tool in order to control the material deposition and to be sure that we can really deposit exactly the same amount all the time. How does that such a process look like it will start thinking on the right side, you can see through the geometry. We have a simplified geometry here, for example, 10 mm. on a structure like a tablet.
This is on target, and on the left side you can see the process diagram how such a process look like. And you have very different parameters that you need to look into. You can see here the melt pressure here in magenta color. This is the melt pressure that we used was around 400 or 430 bar. We have to pressure at another tip running. And now you can see the screw movement and red screw slowly moving forward. Generating this high pressure and you can see then also the dropping frequency in black. You can see. Black, you can see the frequency of how fast does this nozzle move up and down? And you can see we reach our frequency here, about 200 the process here of running about 200, 220, 230 hertz. That is when the infill was done. And then we have a lower frequency here for creating the surroundings here of the tablets, and now you’re in the in the center. You can see the, in red, a screw movement going very high. And so this is in fact we’re watching now the reloading process where the screws are moving backwards. Then we have a rotation of the screw and then refilling this injection volume that we can use later on again for printing. So around 345 seconds here, you can see, again, we continue the printing process. So we were taking about 15 seconds in order to refill the reservoir and then we can continue the process itself. That is graphic illustrating how this concept works, and it really shows how homogeneous it is.
If you look at the melt pressure, it’s very good, controlled the system itself and the droplet frequency very repeatable. But it’s an important part if you look at the 3D printing technology. Yeah, looking at the strategy and how did we implement it here? First of all, we have coming here from the reproducibility. We want to assure the reproducibility of the 3D printing technology, homogeneity of mass and target geometry, that’s the basis to assess this technology. Then an important factor is the mechanical stability. We assessed the mechanical properties here of the dosage forms, focusing rather on the braking strengths and friability, because these are very important parameters that we also see from other 3D printing technologies to, for example, the powder bed systems. Sometimes they have challenges, especially when it comes to friability. Then model compounds, we need a smart selection of model compounds in order to assess here the potential as a drug carrier system and to understand really, how can we modify the release based on the polymeric properties? And we want to achieve at the end, ultimate goal is to achieve an individualization of the release kinetics or of the dosage forms. So I want to create target release kinetics. And how can we do that? How can we really modify it? Very simple for each patient, for example, and we can do that by bringing in the volumes or even force structure.
How does that look like? Here we can see images here of the final geometry you can see here on the top because an SEM image of such a tablet. We choose the tablet with the high porosity because it was a low volume. You only have a 30 percent infill volume for you in order to better understand how these layers are formed. And not on the right side, we are zooming in on a dedicated part of the tablet and you can see that you have here these filaments here consisting out of individual droplets. You can still see that on the right side and on the bottom, you can see the side view on how these layers are aligned on each other. Not the right side again, you can see the zoom. We’re zooming here on to individual filaments that are deposited with the advanced bedrock technology and also, you can see are consisting out of individual droplets and then you create layer by layer. So we can see it is a very homogeneous process, and the important thing is, as we have each droplet defined by this process, we can really assure that each droplet has the same weight and homogeneity. How can we utilize this technology? We can really arrive here infill volumes, and you can see an overview about what is possible.
Here we have a comparison between 30 and a hundred percent of the infill volume, and we can vary that we have the software tools here in order then to just deposit the droplets in a different pattern. And for sure, that leads also to variation of the surface area and the porosity in between. So that can be a nice tool here to vary to target the shapes and the target systems. We did that here just with the Parteck MXP, just a polymer, in order to illustrated for sure. We also evaluated it with our model compounds. How does it look like looking at the mass distributions? That is an important point. First thing for us is really to assure that we can have this sudden reducability with this kind of technology, and now we are comparing the target mass. You can see here the mass and mg plotted versus the different infill volumes. You can see very homogeneous for the placebo 3-D-printed tablets. Often we see a little bit higher deviations, but in total it’s still within the limits of Pharmacopeia. And also ketoconazole loaded tablets. We’re working pretty well, so with all of the tested substances.
It’s very easy to achieve your target and mass distributions that is an important point here and to reach this high reproducibility. Another thing you need to consider here is also the mechanical stability, also an important factor. Now we are looking here at a diameter of compression. So we just compressed the tablets diametrally. We’re a texture analyzer. And then we were detecting the tablets force here in order to break that up. Now we can see the force you plotted against the volume and you can see that even for the lowest interval, even for thirty percent infill only, you can see, for example, the we very fast reached 100 Newton and are even above 300 Newtons. Then, with a higher increase of the infill volume, you can see the braking force even further increases. And then at 600 Newton, we already reached the limit of the testing device. So you can see it’s very stable tablets already at very low infill volumes. That is very good for this kind of technology.
What’s this another effect? You can see the placebo tablets here in black. They reach already refers to maximum and the maximum force of the testing device from 50-60 percent. And you can see, even here even stabilizing these mechanical forces even more. Whereas ketoconazole, for example, here goes plasticizing in effect, so we delay a little bit the maximum. Doesn’t really matter for the stability, as we already at very high levels. But it’s interesting scientifically to to understand how the additions of different drug substances work here, especially looking at the transformation of mechanical properties. You also assessed the friability because for sure, it can be very strong stable, but could be still providing high friability. That is something we needed to take care of. That’s why we checked also for friability. But you can see with all the evaluated systems, we are still far below the recommended one percent from the pharmacopoeia. So that means we are still here safe looking at the viability values and we can confirm the mechanical strength here and for our ability is always an important factor, especially if you think about further processing steps you want. For example, at coating step or packaging step. At the end, you need to have a certain stability and that is also given you with this technology. Most importantly, a legitimate goal here is to utilize this technology for modifying drug, release the drug, creating easier and to adapt it here to the personalized needs of the patients. And what we can see here is coffein released here in percent, always a 10 percent loaded matrices, and we can see the coffein drug substance in black as a reference.
You can see the coffein. It’s very good soluble. So it has a fast onset direct release and then we can greatly reduce here depending on the infill volumes you could see. Now we have a range of porosity that’s we saw before the scanning electron microscope image of images. Here we were writing the porosity between 30 and 100 percent. And we can see the effect now on the release rates. So that means we can really follow individual release rates here, depending on the individual. Right side, you have the same data set just in relation to the targeted MG loading. Yeah, another important thing we want to look at now. We were looking at the drug release of a good soluble component, so that is for us a good driver in order to understand how do these systems really release out of the polymer. But now I just want to dive deeper here, dive deeper into the solubility enhancement, it says, because that is an important technology that we can combine with the 3D printing in order to create additional value. And here were choosing the ketoconazole as a model compound. And see the structure here. It’s a weak base here, BCS class 2 compound with the low sort of political.
And on the left side here, you can see the activity patterns here plotting here, the intensity versus two different. And you can see now you’re the crystal in pattern of the model compound. So we have a very crystalline drug substance. And we also see the physical, premature and blue sort of pre-mixed here of our polymer with the drug substance suitable for shrewdest crystal patterns of the drug itself. And now it is 3D printed tablets in magenta. They don’t show any patterns anymore that are linked to the drug substance, though. We can really see that the drug substance here is converted from a crystalline to its amorphous form to hollow that you can see here, it’s really just linked to the PVA. So that is a big success. You can create ASD with this kind of technology. But now we also want to see how can we utilize it for solubility enhancement and this is what you can see here and see some dissolution curves here where we plot again. Itraconazole amount dissolved here versus the time, and you can see as a reference, we also added the ketoconazole. We choose to make some loading in 40mg. And you can see it doesn’t get into solution solution here. Yeah, it’s very, very low.
Whereas for the 3D printed structures, then we can really enhance the solubility. Also, even depending on infill, we can increase it and then at a higher end for about 70 to 100 percent for sure. We have a certain delay of the onset because you have lower surface area. But then we also reach very high super saturation concentrations and they are even maintained over over an extended time period. That is also an important message here with the PVA with the Parteck MXP. We are really able here to create super saturation and then maintain it. That is an important feature of the polymer itself. You don’t see that with so many polymers. Usually, then the precipitation already starts and we have a re-crystallization of the drug substance. Yeah, so coming to short summary here of the advanced melt-drop deposition and its application for pharmaceutical industry. We see a very new technology that provides the very high accuracy, especially if you look here at the material deposition. So it’s a new technology that really focuses on the material deposition in contrast to other technologies here. We have a very defined process and assume, as you could already see, the process itself is very stable and can be monitored. I think that is a key advantage here. And it gives us the opportunity to combine additive manufacturing with solubility enhancement, so that can really be the key feature here in a new product development.
Another point here, this differentiation to existing FDM technologies, because here we are looking at the single melting step here, that is based here in the direction of direct extrusion, and we can have very complex forms as we have this high defined maturity position out of individual droplets. So that is also an important differentiation. The technology, the technological status here, we see it as an advanced technology that is already pretty much established in plastics industry and we see it fast expanding in other technological fields. And now we could also address this technology of the future of pharmaceutical applications. Looking a little bit more into the future. We can foster an … complex geometries you can see already here some examples of what we are able to do with this technology. We have the high level of etched deposition you can see that becomes very important if you create defined structures, especially also, for example, you can have a breaking edge here, then you can create completely other shapes, bullet shapes defined infill or even patterns. And for that really a link even to the brand.
Or you could have a thin layer. A very thin layer interprets here that are already very stable. Or even use a multi-nozzle in order to combine different drug substances or even just combine different release rates with each other. Yeah, another point you. It’s about the future, where can we apply this technology, we can use it for individualization and rapid prototyping, so we know that the personalized medicine will be an important driver for 3D printing technology. But we also see a high interest here in the rapid prototyping and pharmaceutical industry, and especially also in the early supply for clinical trial material and key technologies, itself here. Due to its simplicity, the FDM process here can be an enabling technology for early formulation development.
But we see two main advantages here also targeting direct extrusion approaches. That is really where we see a lot of companies are exploring. Looking at the growth potential, we see that 3-D printing is gaining very high interest within all industrial sectors, actually. And an important factor that will drive the development and pharmaceutical industry is that the whole pharmaceutical industry is faced with accelerated development timelines. So there will be high demand here for new manufacturing technologies. And one of them will be additive manufacturing because it really has the potential here to speed up, especially the early phases of development. Also, polymer requirements are an important topic here, we see the high thermal stability for the melt based system that is also key. It’s a key requirement here and that’s why we chose the PVA here as a very linear polymer, very stable over an extended time. Also, another important point you need to consider are the mechanical properties, especially if you’re looking into into intermediates, and not all processes are already these one step processes that you saw today. A lot of processes still require an intermediate. And that also requires a certain mechanical as well as polymeric stability. Yeah, that’s it for today. Thank you for your attention to be due to answer your questions.
Also interesting: Creation of Filaments for 3D Printing via Hot Melt Extrusion: Evaluating Different Downstream Technologies
Speaker 1: Thank you, Thomas, for your presentation. We will be at the start of the question and answer session. So with that, should I make you the organizer so that you can read out the questions?
Speaker 1: Yeah, sure.
Speaker 1: Okay. OK, now you will see the Questions tab on your questions panel, and then you can read the questions. So delegates, please type in your questions in the Questions tab and … of the questions, and Dr. Thomas would answer them.
Speaker 1: Now, so we have received first question. So the question is for Thomas? So can you please comment on formulation of protein based drug using the FDM? Question is whether FDM more 3D printing can be used for the protein based formulations?
Speaker 2: You put this whole by loping rather or.
Speaker 1: Yeah, for protein, drugs, alcohol, protein type formulations can be used the FDM technology.
Speaker 2: You mean for protein or prototyping? I didn’t understand…
Speaker 1: Protein, biologics.
Speaker 2: yeah, for biologics, it is it is a little bit more complicated, for sure. We also see a evolution in the area of bioprinting. But the FDA says usually it’s rather based on a filament base or the filament itself. And for example, this is melted by the nozzle. And usually this requires high temperatures. So there we see some limitations, especially for biologics self. Just due to the fact of the high temperatures. But there are other printing systems also already established. If you look into bioprinting so that they are working rather than, I would, for example, gel like structures and they don’t require this excessive heat. So those systems can be also interesting for further bioprinting sector in order to to print proteins or biologicals. There’s also a lot of research in this area ongoing, but it’s a little bit deferring from the FDM. So with the FDM, and we’re working at rather higher temperature ranges and rather at the current stage, let’s say rather for this small molecule area.
Speaker 1: OK, so 3-D printing can be possible, but maybe the FDM is not the right choice for the biologics.
Speaker 2: Yeah, absolutely. Yeah.
Speaker 1: Yeah. So we have next question for you of what infill percentage is desirable for enhancing the solubility of the tablet?
Speaker 2: Very, very good question, also, as you as you saw, we are able to vary the geometry, control the porosity by the infill. And that is a very good tool for our release. And as you could see already from the release, especially if you target formulation that wants to create a certain solubility enhancement and maintain this high solubility over time, it can be of advantage to also reach a certain release kinetic. Because you can see if the structure overall becomes too dense, for example, it will delay your release slightly, but you can delay your release slightly. And that also modifies to release kinetic of your drug substance. Let’s say, for example, most of the polymers let’s say, will be diffusion based, maybe sometimes you would have a mix of diffusion and erosion, but you can imagine that at the surface you would have a slightly up concentration. The concentration of the drug substance then will increase at some point. And so it can be also very interesting to really have to target release speed. So let’s say release kinetic in order to further support your solubility enhancement, because then you really reach a good limit of then releasing your drug and not to accumulate it on the surface area because that may induce crystallization again so that there could be an optimum also for this kind of approach.
Speaker 1: So there is one more question, which is this this person has missed the talk somewhere. So he is saying that it is regarding this the infill volume again. So is any scaffold needed to deposit the materials of what is the material which can be used to make the scaffold. So the question is whether the scaffold is required or if yes, then which of the metal we can use for the scaffold?
Speaker 2: In fact, we can directly use the final melt. So we are just using the final melt and then we can override a geometry is usually we just have an outer layer. Stabilize and then we have the infill pattern that we just followed side wise. So that is the standard pattern that we are using and sometimes also at the bottom. You can print on one layer in order to then easier, easier remove it from from the from the flow structure, but it’s not not even required with a polymer so. But there is very different options possible, but we can work with the standard geometries.
Speaker 1: So, for example, if you made to exclude extruders or and then we can, you are the filaments, and then if you can put into FDM so it’s possible that requires the scaffold, really?
Speaker 2: Absolutely, you can, and you can write this for sure, it has slightly influence also on the release kinetics, how you how you structure it and maybe even under stability. You can optimize that. That is, that’s for sure. There is there’s different types that you can use and it will have an effect. And you can also have certain geometries, but also played with with the with the infill geometries they can for for modify. Also, how fast is your medium entering, how fast it is releasing and that is also geometries you can optimize. But their impact is rather minor to the polymer itself, as it was more interesting. Really focus on the polymer itself and to have an easy tool does much more room to play. Absolutely. There’s a lot of room to play with the geometries, especially.
Speaker 1: There is one more question like this technology are talking about 3-D printing and everything, so the question is from the market perspective. So how many marketed products are dead by using the 3-D printing and how the future looks like? And if you know, the brand then mentioned the brand.
Speaker 2: Yeah. Oh, for sure, yeah. No, no, that’s an important question, because I mean, the the technology evolution will really be driven by the market demand. And as you know, it is kind of a new technology and we see already some applications. I mean, there is already one marketed product, SPRITAM from Aprecia, for example, that is actually this year by powder bed, the jet binding you where you create your form out of the powder bed and then you spray the binder on top and then you have the next powder coming on so really construct it out of the powder. So that is already on the market. And we see also another company that is a very fast evolving year in this field. This Atrios tech, they are using a melt based approach, so they also have a melt deposition where they can really deposit it, and they already have filed already an R&D. So they are also very active in this field, especially in the melt based field. And we see a lot of other companies really looking into this technology field. There’s a lot of companies looking into personalization, especially fabrics, is looking into it, into this kind of evolution. And we see a lot of people being interested in it. And also big pharma companies are interested in it in order to advance their development times and to search for a way how to utilize this technology in their early development. So that is also an interesting part of the applications. And also, we are looking at it’s very versatile from very different angles. Also looking, for example, in the Yeah and the one … technology where we really tried to implement it, for example, as a service for customers to support the early developments. We’re also looking very bright and looking for keeping in perspective, how can we support the pharmaceutical industry and to setting it up with the right security technologies? But first of all, also we’re looking into it as a service provider.
Speaker 1: Thanks, thanks so much for giving the overview about the existing market of product and what exactly technology they are working on now. The next question is from the commercial and production perspective, like what production speeds can be achieved and is this expected easy to commercialize from the commercial perspective? How much easier to implement this technology
Speaker 2: is also a very, very good question because for sure, at the end, we need to have a certain commercialization. And there is different approaches. I mean, there is very little protest currently with this 3D printing. You will have the application, especially as, let’s say, really a pharmaceutical production. That’s the way, for example, that we see if Aprecia is putting it forward is really manufacturing a large scale manufacturing. You can scale it upward was carried out for very high throughput, so it can really meet the market demands. That is a very interesting concept, then if you look, for example, into personalized medications, the idea is rather to to have the FDM enter them that the customers but the enddistributors using it here, for example, the pharmacies or, let’s say, the distribution centers here. They created very personalized. There may be, for example, the FDM and technology can be very interesting. So there will be different technologies for different concepts. And also now what you saw today, the advancement of the position is rather based in the industrial sector because we have the larger equipments, that equipment is rather larger and can be optimized also for a larger throughput, like the structures you saw, for example, today they can be manufactured within one or two minutes and then we have just one another running. So you can have the system, multiple nozzles and you can optimize the speed so that you can have three nozzles in parallel and very simple. And then you can then increase the speed. By that, you can print much more tablets also on one platelet that can be quite automated. And then the idea there would be folks are just scaling it out, sort of upscaling it, and then you can have it run 24/7 and create a lot of material. But just by this, and you have a certain high flexibility for sure in creating your final form and also the locations that is an important aspect. Also an advantage to be considered there with this technology comes.
Speaker 1: And I could see that even in pediatric population as well as dementia, like different sizes down shapes, so that can also be possible for the pediatric population to make them to take the medicine so that the gender, the interest in such a population. Yeah, sure. You’re saying something solid on this.
Speaker 2: I know just looking into into the pediatric population, I mean, that is one of the main drivers. One of the main drivers are really here to to, you know, make life easier for the pediatric population. That is very good motivation also from our end and also for the elderly, really, you know that they take a lot of medications, much more medications than the original. So also there to make the life easier to combine them, that can be a very nice application for the personalized sector. So is a good driver.
Speaker 1: Correct. Absolutely. Now there is one more question, like how the 3-D printing technology can be used to enhance the solubility of particularly soluble drugs.
Speaker 2: Yeah, that is actually coming from the hot-melt extrusion, that’s also where we are coming from technology wise. We have a lot of experience with the utmost extrusion in order to create an amorphous, solid dispersion. So the ASD generation and we are basically transferring the otherwise crystalline molecule into its amorphous form and then disperse it into polymer matrix. And the advantage is really that the drug substance is then not any anymore in a crystalline form. So it is already soluble in the polymer. And then we can we don’t need this energy anymore to overcome the crystalline. The latest energy doesn’t need to be overcome. We have it already amorphous and then it can dissolve together with the polymer. That is a great advantage, as you could see for for the ketoconazole example, we were able here to to enhance and maintain it after this. That is an important point. That’s also the future of the PVA because it was quite unfulfilling. It keeps this low soluble otherwise low soluble molecules, then in solution. As long as in solution. The body can take it in and then you have the bioavailability of the drug compound. That can be another key differentiation in order to to make use of the 3D printing to combine it with the solubility that that would be a big additional value.
Speaker 1: Mm-Hmm. Okay. Yeah, thanks, thanks, Thomas, for giving the heads up like how 3-D printing can also be useful for the poorly soluble drugs. Now the next question is when you compare the conventional tablets and 3-D printing tablets. So is there any difference in pharmacopoeia standards for such type of tablets?
Speaker 2: Yeah. I mean, it is quite a quite a new technology, so we know for sure there are some monographs on the tablets. That’s where everyone is focusing on. Where is your orientation on. And usually we are trying to screen also, how would our technology, for example stand compared to to the standard tableting process, and we can see we are usually reaching almost this level, so so all the requirements that you would have for standard tablets. We can easily … also with this 3-D printing structure. But for sure, it’s not the same for all technologies like some technologies may not provide you enough partners or may not may differ in some angles, so that is vital. There’s also a lot of room for discussion also later on with the with the authorities during the evolution of 3D printing. This is a big regulatory discussion also ongoing. And should they have their own monograph or how does it evolve? And so far, I think the best is to stay close from the tablet monograph as it already provides a lot of insights. But for some dedicated structures, they may not fully comply with 3D, with the normal tablet monograph, but still provide advantages. And that is something where we’re discussing this regulatory bodies are important. And that’s also why we try to be to create a big community here to to have also regulatory bodies looking into it and to exchange with them, because there may be certain advantages that may be blocked by the standard regulation, but could be approached better than. So it’s still an early stage. But with this technology that you saw today, it’s easy to to fulfill the standard requirements of tablets, for example.
Speaker 1: Okay. Thanks, Thomas, for giving that clear indication like there is a possibility to come up with something different for the 3-D printing because the requirements for such type of tablet is quite different than the conventional. So we are looking forward to hear more from the regulatory bodies, how they will think of this 3-D printing dosage form. Now, the question is very similar to what you already answered for the biologics or here, the question is how the 3-D printing technology is feasible for the temperature sensitive product development and commercialization. So maybe the FDM, as you mentioned, FDM technology may not be the right one, but maybe there are different ways that you can operate this technology. But your expert, if you can comment on.
Speaker 2: Yeah, I can. I can go. There is always been the FDM technology that we see a lot of demand for, for looking into lower temperature and can for sure, we need to. Let’s look at those and. We know we’re covering higher temperature range because we wanted to see the additional creation of ASD where we are the formation of ASD that is always good to be at, higher temperatures start to create really the amorphous structure. But there is reasons, as I mentioned, to go to the lower and also and that is something also that is needed here, and that is also something that is important for us also as an excipient provider also to to look into what is the need, what is the what is the need for this kind of technology? Where are the gaps? And there we believe also of lower temperature processing is certainly one of the gaps with the technology that we need to look into. And but especially looking into the biologics mentioned, maybe FDM itself is not the right choice for for biologics, because then you would have these two times heating step that is a little bit complicated sometimes. So there you would rather go into the bioprinting area where you would, for example, print gel structures, something that wouldn’t have this challenge with with the high temperature. But for sure, you have other challenges that you still need to grade your final form and then remove the solvent and maybe try it in a safe environment so that there’s still a complex. But we see a lot of movement in this area, so that can be can be an interesting application within the next year.
Speaker 1: Thanks, Thomas. And there is another question, maybe we can take this as a last question because you are answering so many questions and so please suggest like what are the other polymers have been found suitable to use to read this process, like APF process if there’s was mention. I think it’s going to be a process you want to say, like, what are the other polymers can be found suitable? Yeah.
Speaker 2: Yeah. So we are seeing that usually the polymers, that are used in an hot-melt extrusion are explored. And we are seeing especially for the FDM process for sure, you need to look at the intermediates. So that is something we are also looking into. And actually, some of the polymers used in a hot-melt extruder are designed to be quite printed because for sure, they need to be moved on again. And we see that when we create these filaments. So we also perform a lot of studies where we look into into different polymers and then measure the hardness, measure their mechanical strength. You can do that easily by three point bending, for example, you have it on blocks and then you falls on top and then you can see when do they break? And we’re also working a lot with that. For example, with the PVA, we are using the PVA as a basis. And then really adapting the mechanical properties then to your target. So we are adding plasticizers are those that you can use, for example, polyols, you can use sorbitol, mannitol or something in this direction that gives you a very good results so you can add that small molecules as plasticizer, then you can really design also the flexibility of your strands. And that is that is very important to you. You want to succeed here in the FDM development you to to have that right from the start, you do right mechanical properties. But but you can evaluate different, different problems that are using homemade extrusion well-known polymers. But really, this fine tuning of mechanical properties that you can easily do that with the PVA, that’s an important feature for you to start your early development.
Speaker 1: Yes. So your messages, like with the PVA, you can play around and you can do a lot of changes and still come up with your 3-D printing depending upon your API and depending upon your processes. So maybe you have to do some of the pre formulation study and then you can come up with your next. Welcome, yeah,
Speaker 1: thank you, thank you, Thomas, thank you. Thank you for conducting this Q&A. And before we close this webinar, I will again hand it over to Thomas for his concluding remarks before we close this webinar. Over to you, Thomas, again.
Speaker 2: Thank you. Thank you. Yeah, I think I hope it was a great journey for you today to to see how 3D printing can be utilized for early formulation development. And I mean, we are just at the beginning of this journey, actually. So I mean, we see the first products now, our first companies really focusing on producing medicinal products that are entering the market. So I think it’s really interesting time to to be to be working with this technology, and it is very interesting to connect with with the other people who are working on it to to utilize really the potential for pharmaceutical development. And I think in the future, we will see a lot more applications of the technology, especially in the framework of advanced manufacturing. We all know that FDA and a lot of our regulatory bodies are looking into advancing the technologies here in order to assure supply, to assure drug product safety and additive manufacturing can be an interesting part of this toolbox. And we will see more and more application. We see it in a lot of industries how disruptive it is. Also, especially if you look at automotive or other electronic industries, it’s completely changed the entire supply chain for sure. And pharmaceutical industry, it’s supposed to happen kind of slower because of regulations, but it has the potential really to be a driver for new evolutions, new developments.
Speaker 1: Thank you. So thank you, Thomas, thank you, Dr. Mehta. Thank you, delegates, for taking our time today and being here today. Please do join us for our interesting webinars, which you can see on our website. Our next webinar is on November 18th, which is on multivariate data modeling and process validation. So with this, we are closing this webinar. Good evening and have a nice weekend.