The most recent advances in liquisolid technology: Perspectives in the pharmaceutical industry
Abstract
Hydrophobic drugs exhibit altered bioavailability and pose other challenges at an industrial level due to their poor solubility and dissolution rates. In addition, poor flowability, compressibility, complex dosing schedules, and light-sensitivity problems associated with hydrophobic drugs have led to poor patient compliance. To overcome these problems at an industrial level, the liquid-solid technique is a promising approach for tackling such challenges. This study outlines the prementioned challenges related to hydrophobic drug candidates and introduces the liquisolid technique as a potential alternative using non-volatile water-miscible solvents, carrier agents, coating substances, and their subsequent applications in the pharmaceutical industry. Furthermore, this study highlights the role of liquisolid technology in achieving sustained-release kinetics, emphasizing its benefits in minimizing pH changes in drug release and enhancing photostability. The study aimed to explore the liquisolid technique as an important tool for improving drug delivery, overcoming solubility issues, and optimizing therapeutic outcomes. In addition, this manuscript holds significant importance by highlighting the applications and recent advances in liquisolid technology, focusing on industrial-level applications. Moreover, it is impressive that such a technique offers improved formulation options to enhance the safety and efficacy of therapy. Overall, this study serves as a valuable resource for researchers to overcome formulation challenges and optimize drug delivery in the pharmaceutical industry.
Highlights
- 40% of the newly formulated drugs are hydrophobic having low a dissolution rate and bioavailability.
- Their dissolution rate as well as flowability and compressibility are major challenges at the industrial level.
- Liquisolid technique convert liquid medication to dry, free flowing, non-adherent and readily compressible powder.
- It is more advantageous to controlled release dosage form by achieving zero order release kinetics easily.
- The use of liquisolid technique in photo-stability perfection, minimize the pH triggered variation in the release of dosage forms.
- It has the great potential to be the next generation dosage forms.
Introduction
Oral drug administration is an authentic route of delivery due to its high patient compliance, ease of administration, and cost-effectiveness. However, the main issue with the oral route is the lack of suitable plasma drug levels [1]. Furthermore, to provide the desired concentration of the drug in the systemic circulation, the drug must be present in solution at the gastrointestinal level. Thus, the dissolution rate is a limiting step in the bioavailability of hydrophobic drugs, and poorly water-soluble drugs have incomplete systemic availability [2]. The main challenge for hydrophobic drugs is enhancing their dissolution rates and bioavailability. In the past, different approaches have been employed to increase the dissolution rate of hydrophobic drugs, such as drug solubilization using surfactants [3], and the dissolution rate of ketoprofen has been enhanced using this technique [4]. Celecoxib is linked to a complexing agent that has a higher dissolution rate than its conventional form [5,6]. Crystal engineering [7] and ball milling [8] are other techniques that improve dissolution rates. The dissolution rates of indomethacin and naproxen were enhanced using a ball milling technique [9]. Microprecipitation [10] and solid dispersion [11] are other approaches in this chain. Itraconazole is a hydrophobic drug that has been investigated for enhancing its dissolution rate using these techniques [12]. Cosolvency and salt-formation techniques have been applied to hydrophobic drugs. However, all of these techniques have challenges, and the results obtained are dissatisfactory because fine particles recombine to form agglomerates or clumps. Higher surface energies of the particles and van der Waals forces are the main factors involved in such a scenario [13]. In the case of solid dispersions, commercially available products are limited because the required conditions for the process are not easily obtained. Solid-dispersion techniques involve solvent evaporation and melting. Evaporating the solvent is at times difficult during the solvent evaporation process, which changes the overall product performance. Moreover, the melting process is unsuitable for heat-labile drugs [14]. Ball milling is an expensive technique, and drugs handled using salt-formation techniques are vulnerable to moisture scavenging (hygroscopicity). Furthermore, special adjustments are required for heat dissipation during the process. Dilution of the co-solvent results in drug precipitation [2,[15], [16], [17]]. In microprecipitation, a risk of toxicity from the solvent used (non-aqueous) exists. Wide size distribution is a major issue concerning the microprecipitation technique [18].
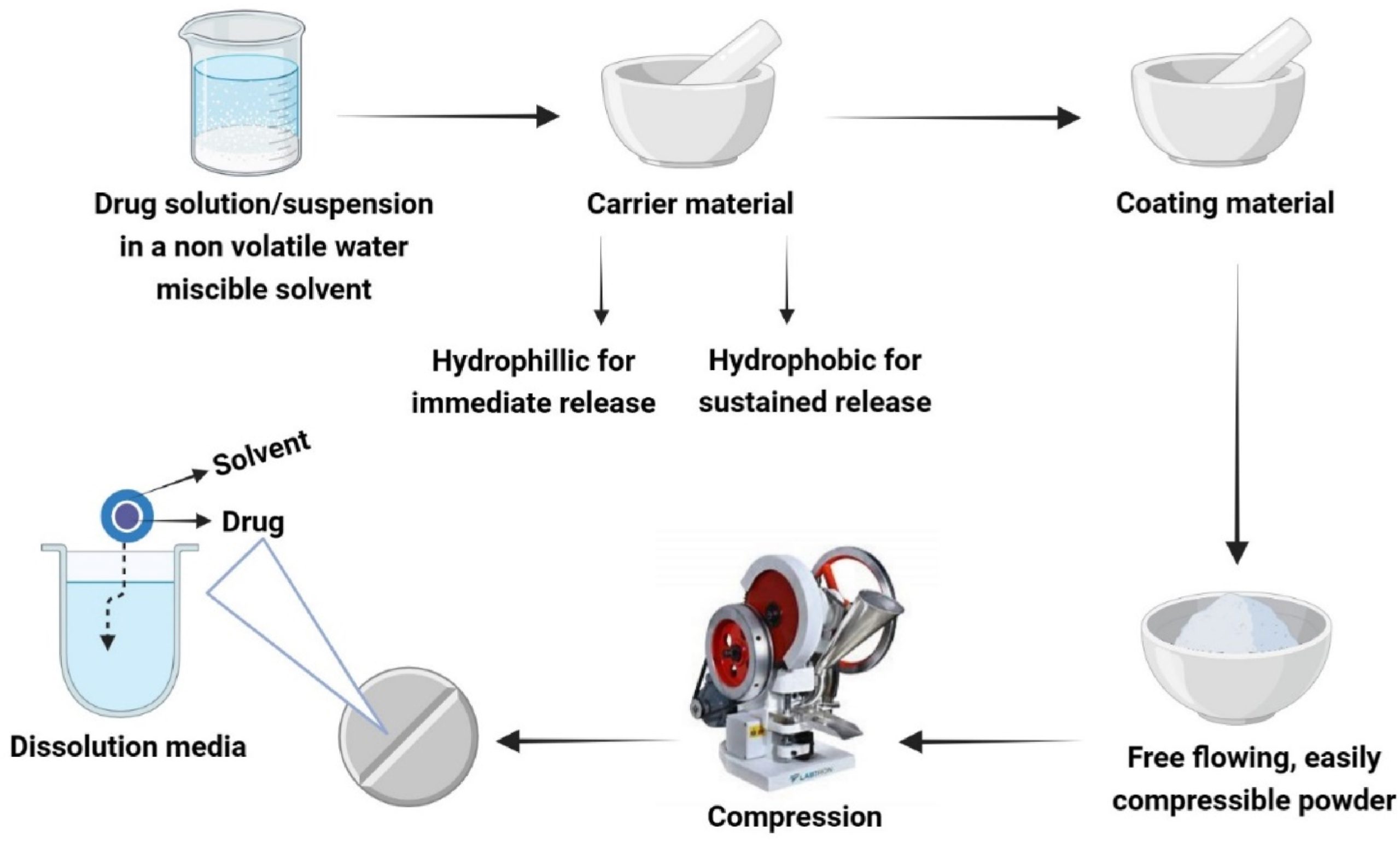
Thus, in the context of the aforementioned drawbacks, powder-solution technology (the liquisolid technique) is a novel and problem-free approach. Powder-solution technology is based on the conversion of a liquid drug (liquid medication) into powder form. This technique involves the introduction of hydrophobic drugs, either dispersed or dissolved in a non-volatile water-miscible solvent, into a specified quantity of excipients (carrier/coating materials). Drying the solvent by absorption/adsorption produces a nonsticky, free-flowing, and readily compressible powder [[19], [20], [21]]. Liquid medication refers to a hydrophobic drug dissolved (solution) or dispersed (suspension) in a non-volatile water-miscible solvent. Occasionally, oily drugs are introduced directly into the carrier and coating material. The carrier materials are porous and have good absorption capabilities. When applied to liquid medication, they provide residence to solubilized drug particles. Commonly used carrier materials are microcrystalline cellulose (MCC) and amorphous cellulose. Drug particles that are unabsorbed by the carrier materials due to saturation are then adsorbed by the coating materials. Coating materials have a high adsorption capacity with a fine particle size and provide free flow to the powder. One such example is silica and its various kinds [22]. A recent study on liquisolid tadalafil tablets showed that when silica was used as a coating material, it provided an angle of slide 33°, which is an acceptable powder flow range. All the other parameters of the powder flow were within the required optimal range. Because good flow ensures the best compression, tablets will be efficiently compressed with accepted physical process parameters [23].
This review article highlights the potential of liquisolid technology to overcome challenges related to hydrophobic drugs, such as poor dissolution rate and solubility, which further create reduced and incomplete bioavailability. Although various approaches have been attempted, scaling up in the pharmaceutical industry has proven to be difficult. This article highlights a few important findings to improve hydrophobic drug bioavailability by improving solubility and dissolution rate. From a formulation perspective, it converts clustered hydrophobic drugs into free-flowing and nonadherent powders, facilitating easy compression. Additionally, patient compliance is crucial, as this technique helps formulate a sustained-release dosage form that overcomes complex and multidosing schedules. Therefore, the liquisolid technique is a promising tool for optimizing drug delivery in the pharmaceutical industry.
Table 1. Preparation of sustained release formulations through the liquisolid technique.
| Drug | Solvent | Carrier material | Coating material |
|---|---|---|---|
| Trandolapril | PEG-400 | HPMC | Colloidal silica |
| Trimetazidine 2HCl | Tween-80 | MCC | Eudragit L100 |
| Tramadol HCl | Propylene glycol | MCC | HPMC |
| Propranolol HCl | Tween-80 | Eudragit L100 | Colloidal silica |
| Griseofulvin | Synperonic PE/L61 | MCC | Cab- O –sil M5 |
| Venlafaxine HCl | Tween-80 | Eudragit L100 | Colloidal silica |
| Theophylline | Polysorbate-80 | Eudragit L100 | Colloidal silica |
Download the full article as PDF here The most recent advances in liquisolid technology
or read it here
Yaseen Hussain, Jinghao Cui, Amos Dormocara, Haroon Khan, The most recent advances in liquisolid technology: Perspectives in the pharmaceutical industry, Pharmaceutical Science Advances, 2024, 100038, ISSN 2773-2169, https://doi.org/10.1016/j.pscia.2024.100038.

