Explicating the Applications of Quality by Design Tools in Optimization of Microparticles and Nanotechnology Based Drug Delivery Systems
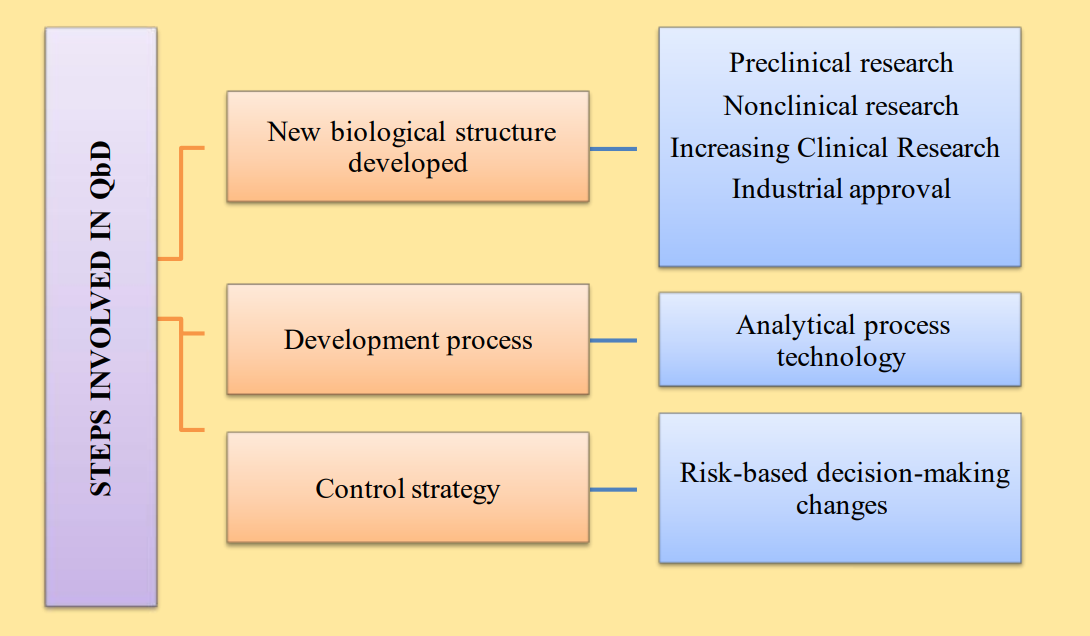
Quality by design (QbD) can also contribute to design, manufacturing, and producing highly finished goods. To better explain the manufacturing processes, the FDA focused QbD in the healthcare industry, based on a comprehensive understanding of how technology and design parameters affect the quality of the manufactured product.
Various elements of QbD are critical quality attributes (CQA); critical material attributes (CMAs), and critical process parameters (CPPs). The tools generally applied in QbD are risk assessment, design of experiments, and process analytical technology. The various benefits of the QbD model are preventing sampling errors and variability in research studies, less experimentation, and enhanced productivity. Since the microparticles and nanotechnology-based formulations need complex experimentation and an extremely time-consuming process, the application of QbD tools in such investigations can intelligently conclude the research processes.
This review article provides a brief outline of the fundamentals, elements, and tools of QbD. Furthermore, the recently published applications of QbD in the optimization of microparticles and nanotechnology-based drug delivery systems have been discussed in this review.
Download the full article as a PDF here
Article information: Neelam Sharma, Sukhbir Singh, Tapan Behl, Nidhi Gupta, Ritu Gulia, Neha Kanojia. Biointerface Research in Applied Chemistry. Volume 12, Issue 4, 2022, 4317-4336. https://doi.org/10.33263/BRIAC124.43174336

