A drug repurposing approach of Atorvastatin calcium for its antiproliferative activity for effective treatment of breast cancer: In vitro and in vivo assessment
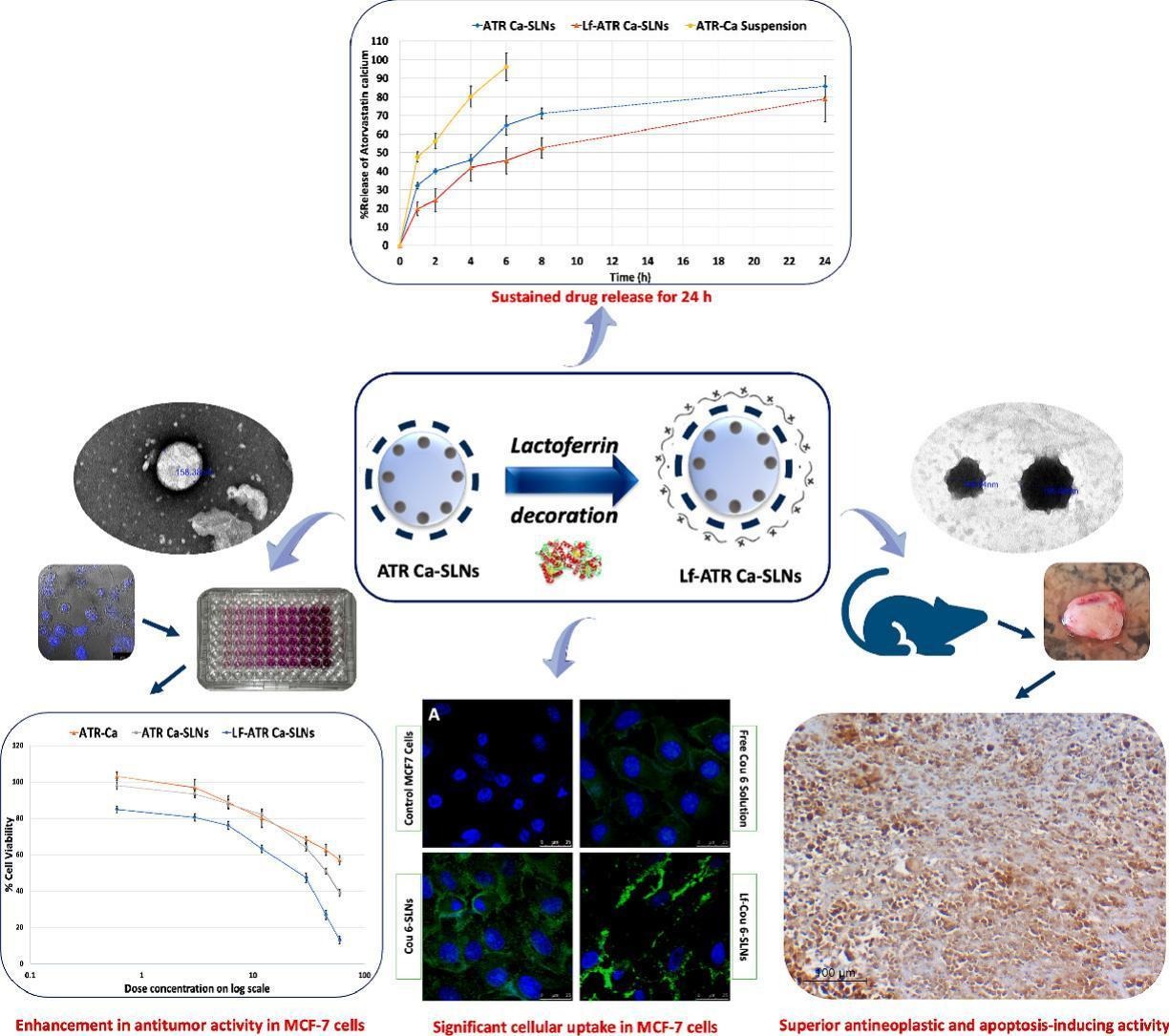
Breast cancer, the most common cancer among women, caused over 500,000 deaths in 2020. Conventional treatments are expensive and have severe side effects. Drug repurposing is a novel approach aiming to reposition clinically approved non-cancer drugs into newer cancer treatments. Atorvastatin calcium (ATR Ca) which is used for the treatment of hypercholesterolemia has potential to modulate cell growth and apoptosis.
The study aimed at utilizing gelucire-based solid lipid nanoparticles (SLNs) and lactoferrin (Lf) as targeting ligand to enhance tumor targeting of atorvastatin calcium for effective management of breast cancer. Lf-decorated-ATR Ca-SLNs showed acceptable particle size and PDI values <200 nm and 0.35 respectively, entrapment efficiency >90% and sustained drug release profile with 78.97 ± 12.3% released after 24 h. In vitro cytotoxicity study on breast cancer cell lines (MCF-7) showed that Lf-decorated-ATR Ca-SLNs obviously improved anti-tumor activity by 2 to 2.5 folds compared to undecorated ATR Ca-SLNs and free drug.
Further, In vivo study was also carried out using Ehrlich breast cancer model in mice. Caspase-3 apoptotic marker revealed superior antineoplastic and apoptosis-inducing activity in the groups treated with ATR Ca-SLNs either decorated/ undecorated with Lf in dosage 10 mg/kg/day p < 0.001 with superior activity for lactoferrin-decorated formulation.
Download the full article as PDF here A drug repurposing approach of Atorvastatin calcium for its antiproliferative activity for effective treatment of breast cancer
or read it here
Materials
The sample of atorvastatin calcium, which had a purity of 99.56%, was generously provided by Pharaonia Pharmaceuticals in Alexandria, Egypt. Free samples of lipids, namely Gelucire 50/13 and Compritol 888 ATO, were obtained from Gattefosse in Lyon, France. Lipoid GmbH, located in Ludwigshafen, Germany, provided soybean phosphatidylcholine with a purity level above 96%. Polysciences Europe GmbH, located in Hirschberg, Germany, provided the substance Coumarin-6. The blue-fluorescent stain Hoechst 33342, which is used specifically to stain DNA (i.e., the nuclei of eukaryotic cells), was obtained from Thermo Fisher Scientific in the United States. The lactoferrin sample was generously provided by Sigma Aldrich, in Schnelldorf, Germany. O-phosphoric acid and acetonitrile (HPLC grade) were obtained from Merck in Massachusetts, USA. The potassium dihydrogen phosphate was supplied by El-Nasr Pharmaceutical Co. in Cairo, Egypt. The MCF-7 breast cancer cell line was acquired from the Centre of Excellence for Regenerative Medicine and Its Applications, Faculty of Medicine, Alexandria University, Egypt. The Dulbecco’s modified eagle medium (DMEM) was acquired from Sigma-Aldrich Corporation, located in St. Louis, Missouri, USA. The fetal bovine serum (FBS) was received from Biowest, which is located in Nuaillé, France. The procurement of Penicillin/Streptomycin was done from Life Technologies, located in Carlsbad, CA. The immunohistochemistry analysis utilized kits and antibodies, including Hydrogen Peroxide Block, Biotinylated Goat Anti-Polyvalent, and Streptavidin Peroxidase, which were procured from Thermofisher Biochemicals, USA. The sodium citrate was acquired from Elgomhoria chemicals, located in Egypt. The Rabbit Polyclonal Antibody Caspase 3 (CPP32) Ab-4 (5 μg/mL) was obtained from Neo Markers-Lab Vision Corporation, USA. Skytek Laboratories provided DAB Plus Chromogen and DAB Plus substrate.
Dina M. Gaber, Sherihan S. Ibrahim, Ashraf K. Awaad, Yasmine M. Shahine, Salma Elmallah, Hebatallah S. Barakat, Noha I. Khamis, A drug repurposing approach of Atorvastatin calcium for its antiproliferative activity for effective treatment of breast cancer: In vitro and in vivo assessment, International Journal of Pharmaceutics: X, Volume 7, 2024, 100249, ISSN 2590-1567, https://doi.org/10.1016/j.ijpx.2024.100249.
Read more on “Lipid Nanoparticles“ here:


