Fabrication of bilayer tablets using hot melt extrusion-based dual-nozzle fused deposition modeling 3D printing
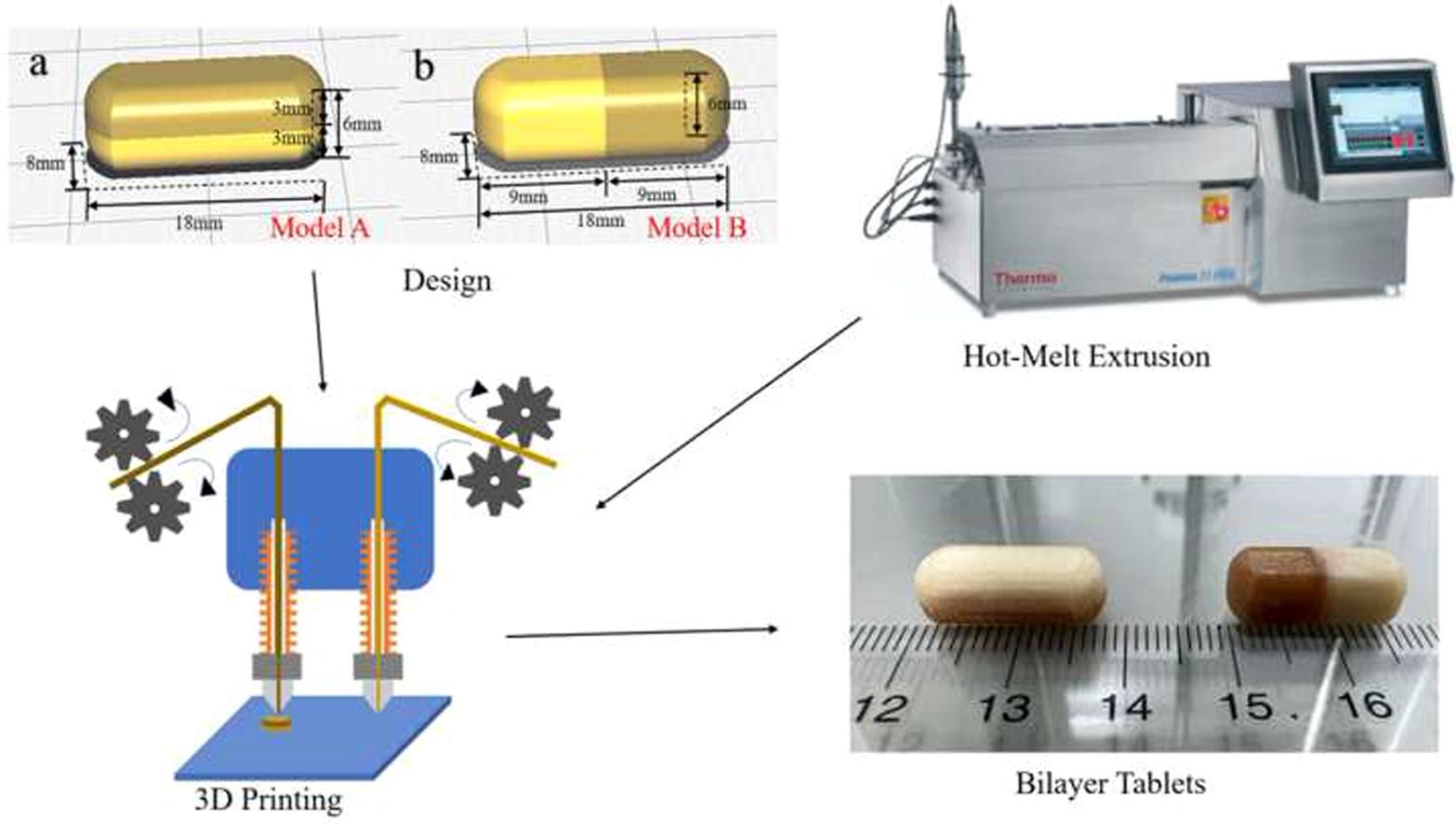
The objective of this study was to fabricate bilayer tablets using hot-melt extrusion (HME)-based dual-nozzle fused deposition modeling (FDM) three-dimensional (3D) printing techniques. Acetaminophen (APAP) and caffeine citrate (CC) were used as the model drugs. Five bilayer tablets with different formulations were developed and two different structures were printed for each formulation. Three-point bending, Hooke’s law, and resistance and stiffness tests were conducted to determine the mechanical properties of the filaments. A novel method, 3D printed tablet retention rate, was developed and used for the first time to compare the printing quality of different filaments. The 3D printed tablets were evaluated to derive the drug release rates using a USP-II dissolution apparatus. HPMC HME 15LV and HPMCAS-LG were identified as good printing materials; however, HPMC HME 100LV was not suitable for printing under frequent nozzle switching conditions. Although mechanical characterization tests can be used to determine whether filaments can be printed, they cannot specifically distinguish the quality of printing between the filaments. Overall, this study revealed the successful fabrication of bilayer tablets via HME paired with dual-nozzle FDM 3D printing.
Peilun Zhang, Pengchong Xu, Sooyeon Chung, Suresh Bandari, Michael A. Repka,
Fabrication of bilayer tablets using hot melt extrusion-based dual-nozzle fused deposition modeling 3D printing,
International Journal of Pharmaceutics, Volume 624, 2022, 121972, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2022.121972.

