Enhancing breast cancer treatment: Comprehensive study of gefitinib-loaded poloxamer 407/TPGS mixed micelles through design, development, in-silico modelling, In-Vitro testing, and Ex-Vivo characterization
Abstract
Breast cancer continues to pose a substantial global health challenge, emphasizing the critical need for the advancement of novel therapeutic approaches. Key players in the regulation of apoptosis, a fundamental process in cell death, are the B-cell lymphoma 2 (Bcl-2) family proteins, namely Bcl-2 and Bax. These proteins have garnered attention as highly promising targets for the treatment of breast cancer. Targeting the overexpressed anti-apoptotic Bcl-2 protein in breast cancer, Gefitinib (GEF), an EGFR (Epidermal Growth Factor Receptor) inhibitor, emerges as a potential solution. This study focuses on designing Gefitinib-loaded polymeric mixed micelles (GPMM) using poloxamer 407 and TPGS (D-alpha tocopherol PEG1000 succinate) for breast cancer therapy. In silico analyses unveil strong interactions between GEF- Bcl-2 and TPGS-Pgp-2 receptors, indicating efficacy against breast cancer. Molecular dynamics simulations offer insights into GEF and TPGS interactions within the micelles. Formulation optimization via Design of Experiment ensures particle size and entrapment efficiency within acceptable ranges. Characterization tools such as zeta sizer, ATR-FTIR, XRD, TEM, AFM, NMR, TGA, and DSC confirms particle size, structure, functional groups, and thermodynamic events. The optimized micelles exhibit a particle size of 22.34 ± 0.18 nm, PDI of 0.038 ± 0.009, and zeta potential of −0.772 ± 0.12 mV. HPLC determines 95.67 ± 0.34% entrapment efficiency and 1.05 ± 0.12% drug loading capacity. In-vitro studies with MDA-MB-231 cell lines demonstrate enhanced cytotoxicity of GPMM compared to free GEF, suggesting its potential in breast cancer therapy. Cell cycle analysis reveals apoptosis induction through key apoptotic proteins. Western blot results confirm GPMM’s ability to trigger apoptosis in MDA-MB-231 cells by activating caspase-3, Bax, Bcl-2, and Parp. In conclusion, these polymeric mixed micelles show promise in selectively targeting cancer cells, warranting future in-vivo studies for optimized clinical application against breast cancer.
Introduction
Breast cancer is a prevalent disease among females worldwide with approximately one million cases recorded annually. These malignancies are difficult to be treated because of their high mortality rates and the substantial costs involved with their treatment(Bhavana et al., 2022, Rajana et al., 2022). However, chemotherapies are associated with significant toxicities(Raju et al., 2024). Molecularly, breast cancer can be divided into subtypes Luminal A, Luminal B, human epidermal growth factor-2 (HER-2) positive, normal breast-like and triple negative breast cancer (TNBC)(Samia et al., 2024). Luminal A breast cancer is the most prevalent subtype accounting for 40% cases. It expresses estrogen receptor (ER) and progesterone receptor (PR) but lacks HER-2 expression. Its prognosis is better than most other sub-types of breast cancer and is commonly approached by endocrine therapy for treatment (Prat et al., 2013). Luminal B subtype accounts for 20% cases and is prognostically poor than Luminal A. Like Luminal A it is positive for ER and PR expression but may or may not express HER-2. Correspondingly Luminal B type is characterized by high levels of Ki-67 protein (Creighton, 2012). HER-2 subtype is marked by lack of ER and PR expression while demonstrating substantial expression of HER-2. It is responsible for less than 15% of the total breast cancer cases. It is characterized by poor prognosis with chemotherapy being adopted in its management. Normal-breast like cancer is similar to Luminal A subtype in terms of receptor expression with only distinction being the decreased levels of Ki-67(Creighton, 2012). TNBC is the most challenging subtype due to lack of therapeutic targets of specific nature, high heterogeneity and poor prognosis with high prevalence among black and pre-menopausal women(Peshkin et al., 2011) (Lee, 2023). TNBC does not express either of the two hormonal receptors that are ER and PR and show absence of HER-2 overexpression. This insensitiveness renders it irresponsive to conventional chemotherapy, endocrine (hormonal) therapy and HER-2 targeted therapy. However significant expression of mutated BRAC1, BRAC2, vimentin epidermal growth factor receptors (EGFR), cytokeratin is observed in 80% of TNBC cases (Mehanna et al., 2019).
The pathological factors such as B-cell lymphoma-2 (Bcl-2) receptors, EGFR, ER, PR, and vascular endothelial growth factor receptors (VEGFR), HER-2 are highly overexpressed in various types of tumors, including breast cancer(Mehra et al., 2018, Mehra and Jain, 2015, Patil et al., 2024, Rajana et al., 2023). Despite significant efforts, effective and targeted cancer therapy options remain elusive(Malavia et al., 2021, Maurea et al., 2010, Selestin Raja et al., 2018).
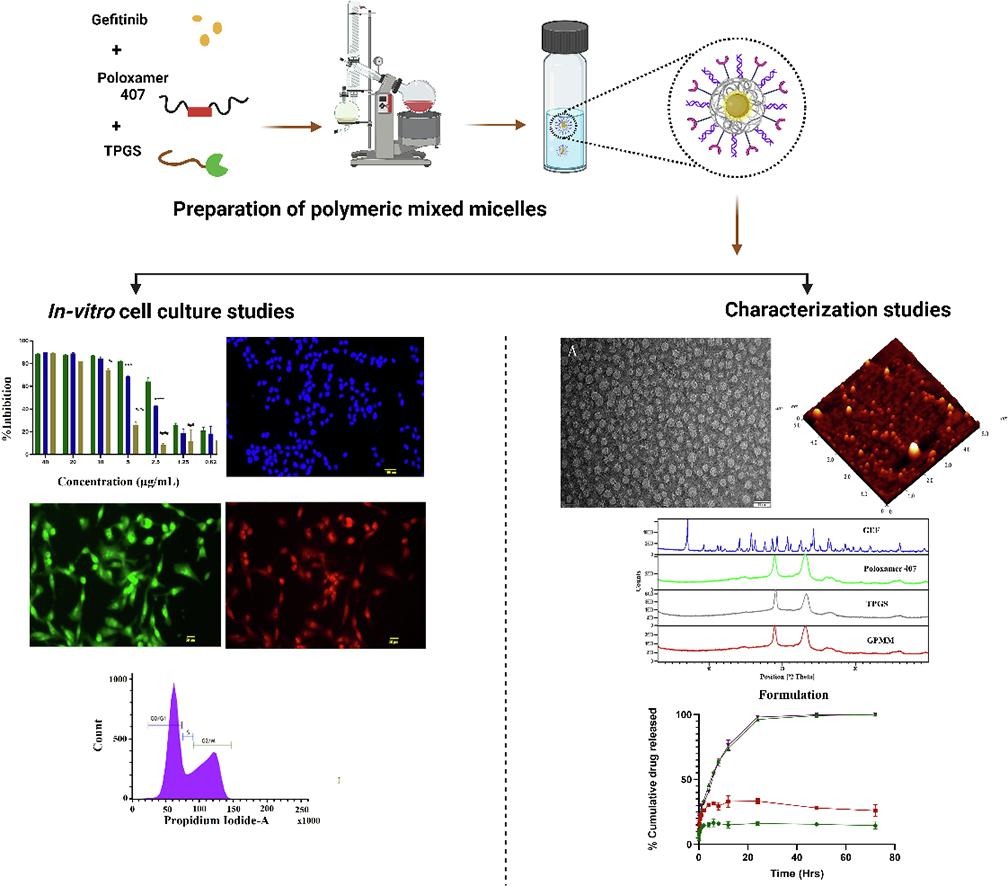
Gefitinib (GEF) is a small molecule EGFR inhibitor approved by the US Food and Drug Administration (USFDA) in 2003 for Non-small cell lung cancer. As EGFR receptors are overexpressed in solid tumors, GEF can prove to be a valuable therapeutic agent in management of breast cancer(Normanno et al., 2006). With a logP value of 4.01 and being in the BCS (biopharmaceutics classification system) class II with low aqueous solubility, currently no pharmaceutical nano-product is available that delivers GEF with an improved anticancer activity. Polymeric mixed micelles are an ideal choice for delivering hydrophobic medications to the target site and are considered the best, most intelligent, and multifunctional nanocarriers for developing pharmaceutical product(Rafael et al., 2018).
The polymeric mixed micelles are an alternative option for safe delivery of the GEF owing to their ability to leverage the enhanced permeability and retention (EPR) effect. The amphiphilic co-polymer, which has hydrophilic and hydrophobic block regions, forms the structural elements of polymeric mixed micelles (PMM). The inner core matter that results from the aggregation or self-assembly of co-block polymer monomeric units typically includes hydrophobic therapeutic molecules. Stable micellar formulations are formed when excipient solutions aggregate and self-assemble at the critical micellar concentration (CMC)(Seow et al., 2007). Various excipients, such as Poloxamer 407, Pluronic® P-123, and Poloxamer 188, are used to synthesize micelles. Poloxamer 407, a biodegradable and biocompatible polymer approved by the USFDA, is utilised due to its monomers, which include hydrophobic groups such as propylene oxide (PPO) and ethylene oxide (EO) units. The fundamental building block of Poloxamer 407 is EO-PPO-EO (Ethylene oxide-Poly propylene oxide-Ethylene oxide), where the PPO group encapsulates hydrophobic medicinal components and the hydrophobic group is surrounded by EO groups(Patil et al., 2021).
To formulate mixed micelles, α-tocopherol PEG-1000 succinate (TPGS), a derivative of vitamin E, is used in addition to poloxamer 407. TPGS acts as a (P-glycoprotein) Pgp-2 efflux pump inhibitor and pro-apoptotic agent, which helps in increasing the drug uptake by cancer cells. Additionally, TPGS enhances the stability of the formulation and improves the entrapment efficiency of the drug(Duhem et al., 2014, Gorain et al., 2018, Koudelka et al., 2015, Zhang et al., 2012).
Micelles, characterized by their small dimensions compared to liposomes and nanoparticles, effectively exploit the EPR effect to target tumor cells optimally(Ibrahim et al., 2022). They are frequently of an adequate size to permit extravasation and aggregation at a tumor location, yet they are typically large enough to escape renal excretion. For instance, cellular uptake studies by Mohan et al. indicated that PEGylated PCL micelles containing DOX exhibited 2-fold and 4-fold higher cellular uptake compared to perfluoro-15-crown-5-ether (PFCE) and perfluoropentane (PFP) nanodroplets, respectively(Mohan & Rapoport, 2010). Moreover, Rapoport et al. reported a 100-fold greater accumulation rate of micelles than nanodroplets in tumor tissue, attributed to the unimer formation upon micelle degradation in human pancreatic cancer MiaPaCa-2 cells(Rapoport et al., 2015).
Micelles have the capacity to increase the solubility, stability, and bioavailability of hydrophobic drugs. pH responsive nature of micelles helps in disassembling the polymeric chains to release drug. Notably, an investigation into the in-vitro release of dexamethasone from both liposomes and micelles revealed a markedly enhanced drug release from micelles under acidic (5%) and neutral (17%) pH conditions over a 14-day period, surpassing liposomal performance (Quan et al., 2014). Furthermore, Poloxamer® 188 micelles demonstrated superior drug release compared to nanoemulsions (Pidaparthi & Suares, 2017).
Additional advantages over other nanosized drug carrier systems include their ability to become fairly structurally stable at large concentrations of the copolymer, (where they form micelles)(Ahmad et al., 2014). The dissociation period following dilution is often longer for these compounds due to their large molecular weights than for other compounds made up of molecules with lower molecular weights(Cagel et al., 2017). These polymeric micelles can circulate in the blood stream for extended periods of time without being detected and subsequently removed when their corona includes PEO chains and additionally exhibit stability in biological fluids(Cagel et al., 2017).
The synthesis of hydrophobic drug incorporated copolymeric micelles is relatively simpler and scalable than other nanoformulations. For the administration of numerous anticancer medications, including as doxorubicin (Dox), ruboxyl (Rb), and paclitaxel in polymer micelles, physical entrapment for delivery to tumor has been achieved(Rapoport, 2007). Compared to several other nanoformulations, micelles are more appealing due to their simplicity of manufacture and biocompatibility. Gefitinib being a BCS class II drug can be efficiently entrapped into the polymeric core of the micelles by varying the ratio of the polymers used to attain maximum drug entrapment(Majumder et al., 2020). Additionally, the versatility of polymer chains and the use of different ratio of the polymers helps modify the drug release pattern to reduce dosing frequency(Husseini & Pitt, 2008).
Micellar drug delivery is regarded safer in administration of hydrophilic as well as hydrophobic drugs through intravenous and oral route as per clinical studies in healthy individuals exhibiting negligible toxicity(Chary et al., 2022). In a comparative analysis between cationic liposomes and micelles conducted by Knudsen et al., liposomes exhibited heightened DNA damage and genotoxicity, even at low doses conversely, genotoxicity was solely reported in micelle-treated groups following prolonged exposure to high doses (Knudsen et al., 2015). Pre-clinical evaluation suggested that tumor regression in MiaPaCa-2 tumor bearing mice was relatively rapid within 3 weeks for paclitaxel (PTX) loaded micelles (PTX-micelles) while PTX-nanoemulsions demonstrated a slower resolution which could be attributed to the enhanced drug release rendered by elasticity in the core PEG-PDLA [poly-(d lactic acid] micelles(Gupta et al., 2015). Based on the extensive literature within the field of nano-drug delivery systems, it can be inferred that micelles serve as a promising drug carrier system due to their smaller size, enhanced cellular uptake, gradual and sustained release, and a comparatively lower toxicity profile. These attributes collectively position micelles as an ideal candidate for targeted cancer therapy.
The main objective of the current study is to develop and assess the polymeric mixed micelles consisting of poloxamer 407/TPGS loaded with the EGFR inhibitor GEF and schematic representation is shown in Figure S1. Numerous physicochemical and analytical methods have been employed for extensive characterization of the GEF-loaded polymeric mixed micelles (GEF-PMM). Stability studies, in-vitro and ex-vivo studies were conducted for further validation of their therapeutic efficacy in development of a pharmaceutical product (nanomedicine).
Read more here
Materials
Gefitinib was provided as a free sample by an Indian pharmaceutical company located in Hyderabad. Poloxamer 407 and α-tocopherol PEG-1000 succinate were procured from a United States-based supplier known as Sigma Aldrich. Cancer cell lines were obtained from the National Centre for Cell Sciences (NCCS), Pune, India. Acetonitrile was procured from Finar Chemicals Ltd Gujarat, India.
Padakanti Sandeep Chary, Ankush Bansode, Naveen Rajana, Valamla Bhavana, Siva Singothu, Anamika Sharma, Santosh Kumar Guru, Vasundhra Bhandari, Neelesh Kumar Mehra, Enhancing breast cancer treatment: Comprehensive study of gefitinib-loaded poloxamer 407/TPGS mixed micelles through design, development, in-silico modelling, In-Vitro testing, and Ex-Vivo characterization, International Journal of Pharmaceutics, Volume 657, 2024, 124109, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124109.
Watch the video below an read more on Vitamin E TPGS here:

