Design of experiments approach on the compaction properties of co-amorphous tablets
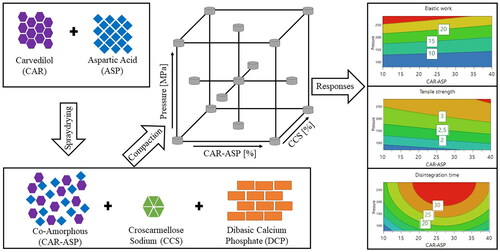
Co-amorphous systems are an evolving strategy to stabilize the amorphous form of a drug molecule with the aim of overcoming its poor water-solubility. With research focussing on the molecular level of co-amorphous systems, little is known about their downstream processing. In this study, tablets of co-amorphous carvedilol and aspartic acid (CAR-ASP) with calcium hydrogen phosphate and croscarmellose sodium as excipients were produced using a compaction simulator. The amorphous form of spray dried CAR-ASP and the subsequently produced tablets was confirmed with XRPD. Over the storage time of 12 weeks, no recrystallization of the amorphous material was observed. A central composite face-centred design with three factors was set up to investigate the interplay of formulation and processing variables with the tablet characteristics elastic work, tensile strength and disintegration time.
As a result, increasing the amount of co-amorphous material led to a decrease in elastic work and an increased tensile strength. These effects were beneficial for tablet properties, namely harder tablets and reduced elasticity. Disintegration time was prolonged by amounts of up to 25–30% co-amorphous material, while larger amounts induced faster tablet disintegration. While showing the feasibility of compacting co-amorphous material with calcium hydrogen phosphate, this study also gives insight into how tablet characteristics are affected by co-amorphous material and relevant process parameters.
Read more here
Design of experiments approach on the compaction properties of co-amorphous tablets, Florian Engelsing, Laura Buchart &Holger Grohganz, Received 09 Aug 2023, Accepted 19 Oct 2023, Published online: 23 Oct 2023, https://doi.org/10.1080/10837450.2023.2274390
Read more on Disintegrants – Pharmaceutical Excipients here:


