Three-dimensional (3D) printing of oral dental films (ODFs) using blended Compactcel® polymers through semi-solid extrusion (SSE) bioprinter
Abstract
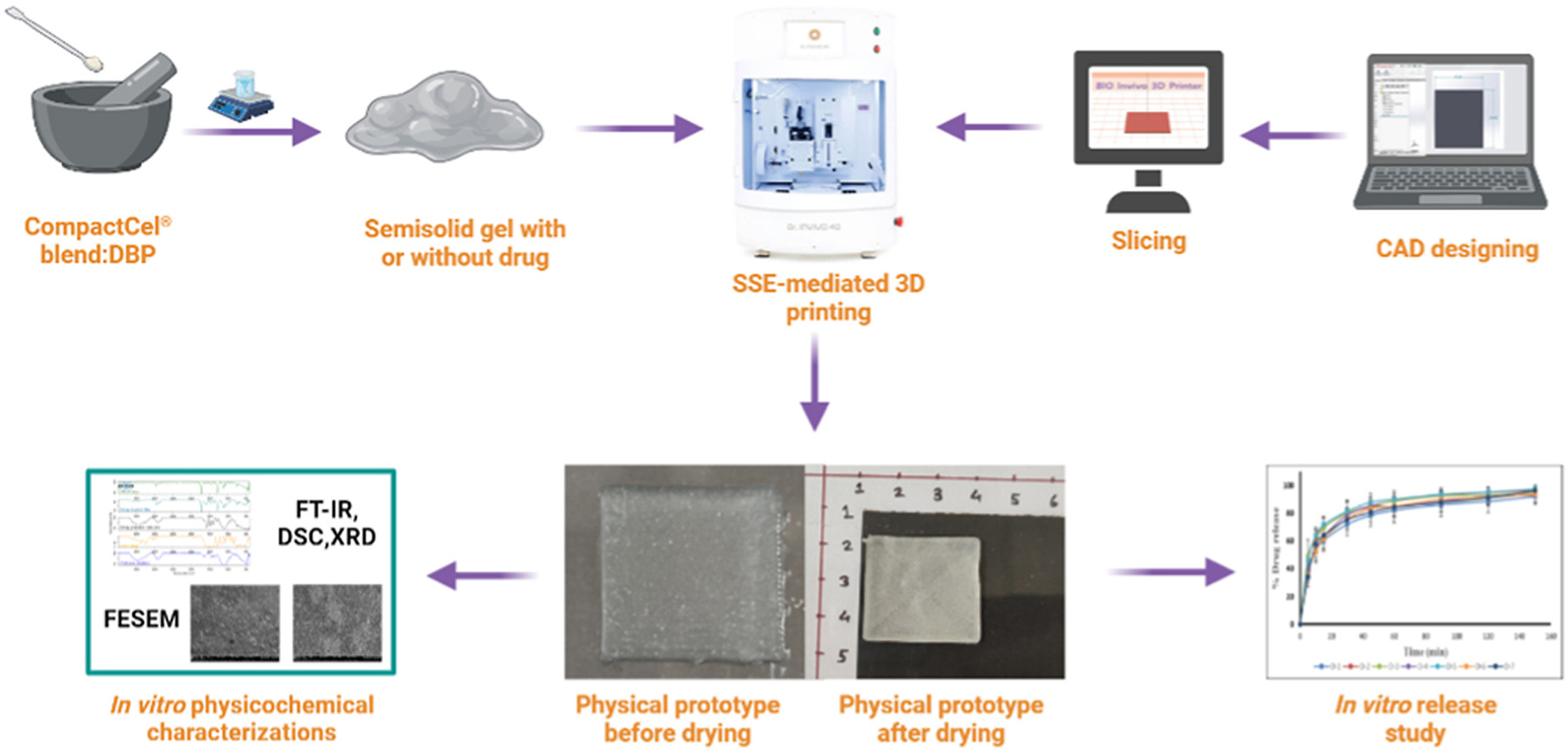
This study aimed to prototype oral dental films (ODFs) loaded with diclofenac sodium (DS) using two different grades of CompactCel® polymers through a semi-solid extrusion (SSE) bioprinter. This three-dimensional (3D) printed ODFs were developed for the treatment of toothaches with immediate and sustained release features. Two different grades of CompactCel® polymers, CompactCel® P 002.02 SR and CompactCel® P Clear 194.04 SIL, with sustained and immediate release features, respectively, were explored in this study. A blend of CompactCel® polymers was found to be capable of forming hydrogels with the addition of dibutyl phthalate (DBP) as a plasticizer to improve the foldability/flexibility of the developed ODFs. ODFs were 3D printed using an SSE bioprinter by varying the amount of DBP. All 3D bio-printed ODFs were analyzed systematically in terms of in vitro physicochemical characteristics, including drug content, drug release, and release kinetics models. The in vitro release graph of DS from ODFs showed an initial burst release of around 50–70% in 20 min followed by the sustained release of up to 150 min for all the formulations. The prototype ODFs showed dual drug delivery features in terms of initial fast release followed by sustained release. Thus, these ideal biomaterial combinations were explored for the first time to establish not only their pharmaceutical 3D bioprinting capabilities but also their potential for drug delivery applications.
Introduction
Three-dimensional (3D) printing technology has the potential to produce a paradigm shift in the conception, manufacture, and utilization of medications by end users [1]. 3D printing is a unique method for speedy prototyping that implies the sequential deposition of several layers to generate solid objects [2,3]. The introduction and use of 3D printing have triggered massive innovation in diverse areas such as aerospace, architecture, bio-fabrication, tissue engineering, biomedical research, and pharmacy [[2], [3], [4]]. The oral route is the most popular and effective way to administer medications because it is less expensive and has better patient compliance, flexible formulation, and improved bioavailability [[5], [6], [7], [8]]. Among the various techniques deployed in the 3D bioprinting process, the semi-solid extrusion (SSE) technique involves gel or paste extrusion through a printing nozzle (with or without the involvement of a drug) without deploying any temperature that quickly hardens the extruded biomaterials and acts as a foundation for the subsequently deposited layer. SSE is devoid of any photoreactive/photocrosslinkable polymer solution exposed to ultraviolet (UV) for stereolithography (SLA)-mediated 3D printing or filament extrusion-driven fused deposition modelling (FDM) 3D printing involving high temperatures, which may cause serious challenges such as drug degradation, free radical generation, prototype stability, and complexity of the SLA and FDM printing processes [[9], [10], [11], [12], [13], [14], [15]]. To mitigate these challenges, an SSE-mediated 3D bioprinting process that could be printed at room temperature was adopted. The SSE-mediated bioprinting process offers a wide variety of drugs, polymer/gum, and plasticizing agents, along with their varied concentrations, to bioprint and ensure the uninterrupted delivery of drug-containing biomaterials without the introduction of any organic solvents with reliable drug release patterns [[16], [17], [18]].
A 3D bioprinter was also used for prototyping thermosensitive pharmaceuticals to avoid the issue of drug, excipients, and/or prototype degradation because it does not require much temperature; rather, room temperature is sufficient to prototype the extruded materials. In addition, various pharmaceutical semi-solid masses are easily accessible for the production of precursor materials. Because of aqueous-solvent-based printing formulations, a drying phase is required to produce solid products [19,20]. A gel was used as the material loading mechanism for the SSE bioprinting. During the operation, a syringe containing the material (with or without the involvement of the drug) was squeezed under pressure and the gel was dispensed via a printing nozzle. The gel was prepared by combining the drug with pharmaceutical additives using a straightforward technique, except for drying and chilling, and no additional post-processing was required. Temperature-sensitive or ultraviolet (UV)-sensitive drugs can be deployed during SSE bioprinting as they do not require high temperatures or UV-based photocuring phenomena [21].
Thus, this study aimed to prototype diclofenac sodium (DS)-loaded and unloaded ODFs using different grades of CompactCel® polymers to explore its pharmaceutical additive manufacturing potential through SSE-mediated 3D bioprinting technology that can deliver DS in a dual release manner (i.e., immediate and sustained), which will suffice both technological and delivery application perspectives of the developed prototype to provide relief against toothaches.
Read more here
Materials
CompactCel® P 002.02 SR (Carmellose sodium, Hypromellose) and CompactCel® P clear 194.04 SIL (Carmellose sodium, Hydroxypropyl cellulose, Silica colloidal hydrated) were kindly gifted by BIOGRUND (GmbH, Germany). Dibutyl phthalate (DBP) was procured from Sigma-Aldrich (St. Louis, MO, USA). Diclofenac sodium (DS) was obtained from Yarrow Chemicals Pvt., Ltd. (Mumbai, India). Analytical grade solvents and chemicals were used throughout the study.
Rohit Bhawale, Purushottam Suryavanshi, Subham Banerjee, Three-dimensional (3D) printing of oral dental films (ODFs) using blended Compactcel® polymers through semi-solid extrusion (SSE) bioprinter, Bioprinting, 2023, e00287, ISSN 2405-8866, https://doi.org/10.1016/j.bprint.2023.e00287.
Visit our new Webinar:
Solving capping challenges using mannitol as an excipient model
Get more information & register here:


