Control Strategy for Process Development of High-Shear Wet Granulation and Roller Compaction to Prepare a Combination Drug Using Integrated Quality by Design
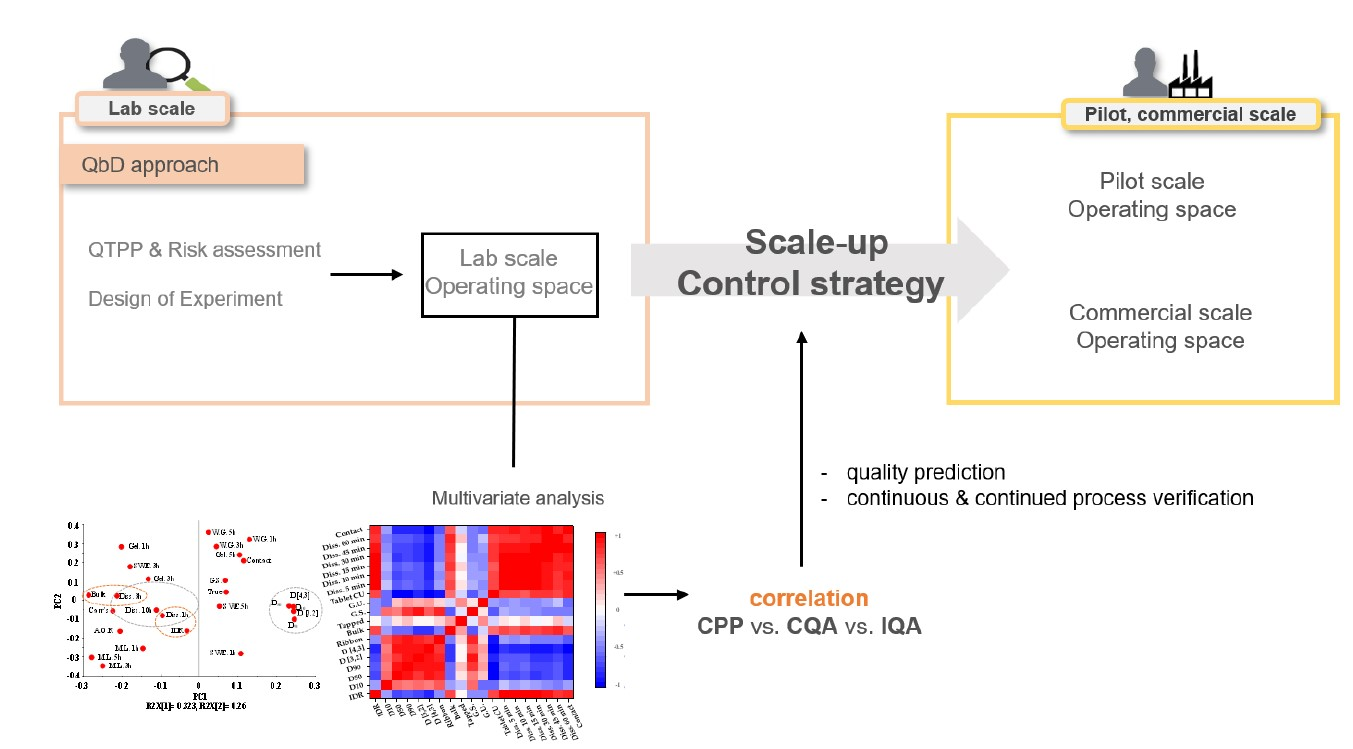
In this study, we developed a control strategy for a drug product prepared by high-shear wet granulation and roller compaction using integrated quality by design (QbD). During the first and second stages, we optimized the process parameters through the design of experiments and identified the intermediate quality attributes (IQAs) and critical quality attributes (CQAs) relationship, respectively.
In the first stage, we conducted an initial risk assessment by selecting critical process parameters with high impact on IQAs and CQAs and confirmed the correlation between control and response factors. Additionally, we performed Monte Carlo simulations by optimizing the process parameters to deriving and building a robust design space. In the second stage, we identified the IQAs and CQAs relationship for the control strategy, using multivariate analysis (MVA). Based on MVA, in the metformin layer, dissolution at 1 h was significantly correlated with intrinsic dissolution rate and granule size, and dissolution at 3 h was significantly correlated with bulk density and granule size.
In dapagliflozin layer, dissolution at 10 min and 15 min was significantly correlated with granule size. Our results suggest that the desired drug quality may result through IQAs monitoring during the process and that the integrated QbD approach utilizing MVA can be used to develop a control strategy for producing high-quality drug products.
Download the full article as a PDF here or read it here
Materials: Metformin and dapagliflozin were supplied from Kyung-dong pharm (Seoul, Korea). Hydroxypropyl methylcellulose (100,000 cps, SR type) and Low-substituted hydroxypropyl cellulose (L-HPC) were purchased from Shin-Etsu Chemical Co., Ltd. (Tokyo, Japan). Lactose monohydrate, magnesium stearate, silicon dioxide, and microcrystalline cellulose were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Calcium silicate was purchased from Fluka (Buchs, Switzerland). All other reagents were analytical or HPLC grade.
Article information: Kim, J.Y.; Chun, M.H.; Choi, D.H. Control Strategy for Process Development of High-Shear Wet Granulation and Roller Compaction to Prepare a Combination Drug Using Integrated Quality by Design. Pharmaceutics 2021, 13, 80. https://doi.org/10.3390/pharmaceutics13010080

