Formulation and Preparation of Losartan-Potassium-Loaded Controlled-Release Matrices Using Ethocel Grade 10 to Establish a Correlation between In Vitro and In Vivo Results
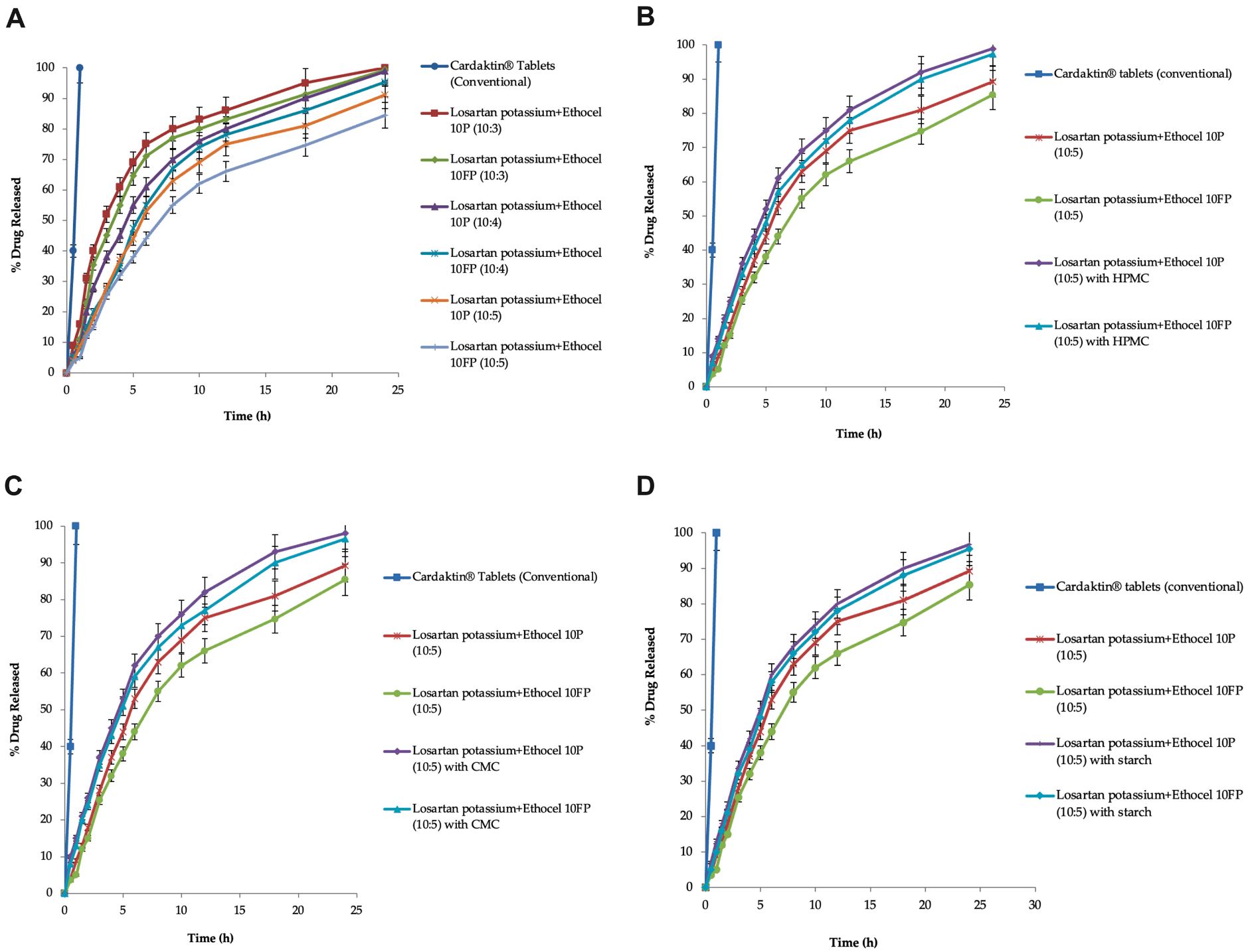
In the current study, matrices of losartan potassium were formulated with two different polymers (Ethocel 10 premium and Ethocel 10FP premium), along with a filler and a lubricant, at different drug-to-polymer w/w ratios (10:3, 10:4, and 10:5). The matrices were tested by the direct compression method, and their hardness, diameter, thickness, friability, weight variation, content uniformity, and in vitro dissolution tests were assessed to determine 24-h drug release rates. The matrices with Ethocel 10 FP at a 10:4 ratio exhibited pseudo-zero-order kinetics (n-value of 0.986), while the dissolution data of the test matrices and reference tablets did not match. The new test-optimized matrices were also tested in rabbits, and their pharmacokinetic parameters were investigated: half-life (11.78 ± 0.018 h), Tmax (2.105 ± 1.131 h), Cmax (205.98 ± 0.321 μg/mL), AUCo (5931.10 ± 1.232 μg·h/mL), AUCo-inf (7348.46 ± 0.234 μg·h/mL), MRTo-48h (17.34 ± 0.184 h), and Cl (0.002 ± 0.134 mL/min). A correlation value of 0.985 between the in vitro and in vivo results observed for the test-optimized matrices was observed, indicating a level-A correlation between the percentage of the drug released in vitro and the percentage of the drug absorbed in vivo. The matrices might improve patient compliance with once-a-day dosing and therapeutic outcomes.
Download the full article as PDF here: Formulation and Preparation of Losartan-Potassium-Loaded Controlled-Release Matrices Using Ethocel Grade 10 to Establish a Correlation between In Vitro and In Vivo Results
or read it here
2. Materials and Methods
2.1. Materials
Well & Well Pharma UK Ltd. (Islamabad, Pakistan) kindly gifted losartan potassium, Ethocel 10 premium (375 µm), and Ethocel 10 FP premium with a particle size of 6.4 µm (Dow Chemical Co., Midland, MI, USA). Magnesium stearate and spray-dried lactose were purchased from BDH Chemical Ltd. (Poole, UK); the co-excipients HPMC (white powder, 88 µm), CMC (88 µm), starch (88 µm), monobasic potassium phosphate, and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cardaktin® tablets (Hygeia Pharmaceutical, Islamabad, Pakistan) were purchased from a local pharmaceutical market as immediate-release tablets containing 100 mg of losartan potassium. Losartan potassium controlled-release tablets were unavailable in the Pakistani pharmaceutical market, so the Cardaktin® tablets were selected as reference tablets for dissolution profile comparisons. Rabbits were purchased from animal houses, GCPS, the Faculty of Pharmacy, Gomal University, DIK, and Pakistan. The chemicals used were of analytical grade and were used without further purification.
2.2. Tablet Formulation
Formulations of the drug’s API (active pharmaceutical ingredient) were established using different grades of Ethocel polymers (Ethocel 10 premium and Ethocel 10 FP premium) with various D:P (drug:polymer) ratios of 10:3, 10:4, and 10:5, and the D:P was taken as weight by weight (w/w). Each controlled-release matrix contained 100 mg of the drug (API), which was kept constant in each matrix. Magnesium stearate 0.5% (w/w) was used as a lubricant, and spray-dried lactose (filler) was used as a filler. Co-excipients such as HPMC, CMC, and starch were added to some selected formulations (D:P of 10:5) by replacing 10% of the fillers. A pilot batch of 110 tablets of each type was formulated. The mixtures of each formulation were made as follows: Each ingredient from each batch was weighed separately using a digital electronic balance (AX-200, Tokyo, Japan). The drug (API) and polymer were mixed using a pestle and mortar. The mixture was added to polybags for further mixing. The filler (spray-dried lactose) was also added, and in the case of matrices with co-excipients such as HPMC, CMC, and starch (10% of the filler), they were also added, and mixing was performed for 15 min. These formulation mixtures were passed through the No. 32 mesh screen, and then the lubricant (magnesium stearate 0.5%) was added, mixed for 10 min, and again passed through the No. 32 mesh screen to ensure uniform mixing.
Khan, K.A.; Ahmad, A.; Marini, C.; Nicotra, M.; Di Cerbo, A.; Fazal-Ur-Rehman; Ullah, N.; Khan, G.M. Formulation and Preparation of Losartan-Potassium-Loaded Controlled-Release Matrices Using Ethocel Grade 10 to Establish a Correlation between In Vitro and In Vivo Results. Pharmaceutics 2024, 16, 186. https://doi.org/10.3390/pharmaceutics16020186
Read more on Magnesium Stearate here:


