Successful drug development with synthetic lipids: critical aspects and strategies
Lipids are gaining enhanced momentum due to their vital role in the field of RNA therapeutics and vaccine development for diseases such as cancer and COVID-19.
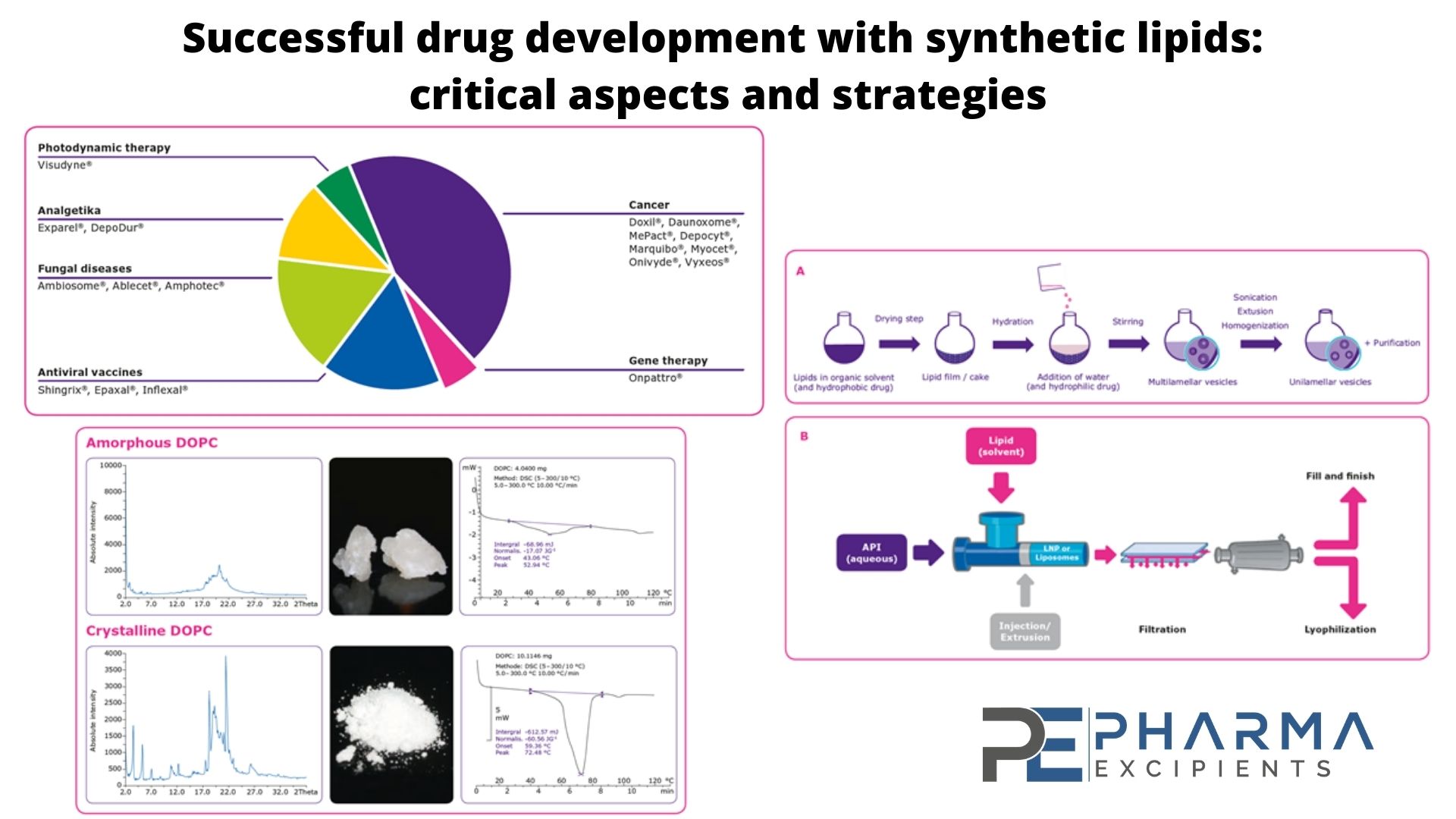
Currently, there are 18 liposomal drugs approved by the U.S. Food and Drug Administration (FDA) and hundreds of lipid based drug candidates in the clinic for a range of ailments1. Lipid-based formulations and lipid nanoparticles have shown promise in drug development and delivery due to their ability to2,3:
- Enhance active pharmaceutical ingredient (API) stability by protecting the API from immune response, proteases and other factors
- Boost the solubility and bioavailability of drugs with poor water solubility
- Passively target inflamed or tumor tissues due to their leaky vasculature
- Improve the toxicity profile of the entrapped API
- Ability to deliver difficult APIs such as RNA, which are prone to instability, nuclease-mediated lysis, strong immune responses, and inability to reach the site of action.
See the short overview here in the video:
The newest advancements in lipid-based drug delivery research and drug development is in the field of nucleic acid delivery, for APIs such as short RNAs for gene silencing or activation (for e.g. siRNA, miRNA, saRNA) and long RNA (mRNA) for applications in cancer therapy, enzyme replacement therapy, vaccines, and more. The first vaccine to enter clinical trials for COVID-19 was a mRNA vaccine, where the mRNA of a viral antigen was encapsulated in a lipid nanoparticle. The success of a RNA vaccine for COVID-19 will catalyse this field leading to the approval of many more gene therapy drugs using lipids in the next few years.
Advantages of Synthetic Lipids
Lipid type, source, and quality/purity have a direct impact on the impurity profile and properties of final liposome formulations, such as the particle characteristics, bilayer structure, stability, and drug-release profile. For reproducible results, it is essential to synthesize lipids using only high-quality raw materials with optimal material characteristics and consistent quality.
Chemically synthesized lipids are advantageous over natural lipids, because they consist of a single lipid of known quality, whereas tissue-derived lipids are usually a mixture of egg-derived or bovine-derived lipids. Unlike tissue-derived lipids, synthetic lipids do not show batch-to-batch variability or risk of viral or protein contamination.
The purity of synthetic lipids can be optimized by selecting high-quality starting materials and optimizing the manufacturing processes and purification techniques. Raw materials should have a low level of by-products, defined stereochemistry (D/L) and isomeric purity (cis/trans), low bioburden and endotoxin levels, and be plant derived with bovine spongiform encephalopathy (BSE)/transmissible spongiform encephalopathy (TSE) and non-genetically modified organism (GMO) certificates and produced using only class II or III solvents. Class I solvents should be avoided based on guidelines from the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) in ICH Q3C.
Material Characteristics and Scalability
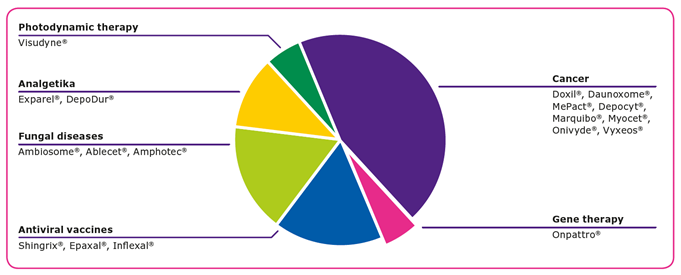
GMP manufacturing of liposome-based drug products requires high and consistent quality raw materials, and characteristics including the solubility, crystallinity, stability, and flowability play important roles in the manufacturing process. Lipids, the key raw materials for producing liposomes, are waxy by nature, which can result in slow dissolution rates and lead to challenges when handling them in large quantities.
Four methods can be used to enhance the surface characteristics of lipids to achieve fast and complete dissolution and enable reproducible liposome manufacturing processes: cryo-milling, spray drying, crystallization, and lyophilization. In addition to solubility improvements, these processing methods provide liposomes with higher purity, enhanced stability, and easier handling characteristics, all of which enable easier formulation. Spray drying and lyophilization result in materials with very high surface areas, high homogeneity, and good handling characteristics. Crystallization is one of the most commonly used methods to enhance the surface of lipids.
Scalability, reproducibility, and optimization of the GMP manufacturing process with respect to liposome yield, concentration, and isomeric purity and other quality aspects, as well as reaction and work-up times, are also paramount for ensuring the greatest efficiency and lowest cost possible. Scalability for both the synthesis and purification operations should be considered from the very beginning to ensure an economy of scale with increasing batch size, with the aim of minimizing the number of synthetic steps and clearly defining them for GMP manufacturing. Ideally, crystallization or liquid/liquid extraction methods should be used for purification, with filtration over silica gel used as an alternative to chromatography whenever possible.
Methods of Liposome Manufacturing
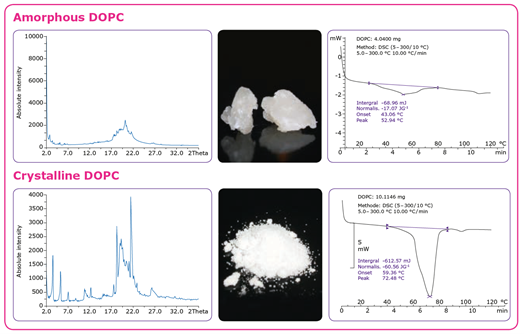
Liposomes or lipid nanoparticles can be manufactured using several different methods, but the challenge is to ensure scalable, robust, and efficient processes4. One method for producing multilamellar vesicles (MLVs) (Figure 3A) is to dissolve the lipid in an organic solvent followed by solvent removal (drying) and hydration with water under agitation. If unilamellar vesicles (ULVs) are desired, they can be generated by adding a sonification or extrusion step to downsize the vesicles. Hydrophobic APIs are added to the solvent during the dissolution step, while hydrophilic drug substances are added to the aqueous solution in the hydration step. Purification is typically the final step in the process. Ethanol injection, in which the lipids are dissolved in ethanol and rapidly mixed with an aqueous medium containing the API, is a prominent alternative method for the production of small ULVs containing hydrophilic compounds (Figure 3B).
The choice of manufacturing method often depends on the final application.6 The ethanol injection method is suitable for the production of small ULVs and stable nucleic acid lipid particles but not for creating large liposomes, such as the larger MLVs and multivesicular vesicles used for vaccines administered by subcutaneous injection or intramuscular injection. In this case, the rehydration method is used.
Developing Lipid-based Drug Products
In the preclinical stages of liposome-based drug development, the optimum, cost-effective synthetic route should be identified, feasibility studies completed, and lab-scale production runs performed. Process optimization should be the focus during the late preclinical stage and phase I clinical trials, including identification of key raw materials and the development of required analytical methods for the lipid. Process scale-up as appropriate should be performed during clinical trials, including implementation of analytical methods and determination of in-process controls, with stability studies also underway. Critical parameters for the process must be defined, process validation of the lipids planned, raw material suppliers qualified, and rigorous risk analysis and intermediates testing completed during late-stage clinical trials. Selecting the wrong raw materials and material suppliers can lead to negative financial implications and delays.
Regulatory aspects for lipid-based drug formulations
There is no clear path to regulatory approval of liposome drug products due to the absence of global harmonized regulatory requirements for lipid excipients. Since the purity and quality of the lipid components can affect the quality of the lipid-based formulation, detailed information on chemistry, manufacturing and controls is requested by regulatory authorities5-19.
Due to the challenging regulatory environments, it is recommended that the drug manufacturer works closely with a supplier that provides regulatory expertise and counsel through all phases of clinical development and commercialization, covering all aspects of quality assurance and documentation.
Conclusion
For successful drug development with synthetic lipids, the GMP manufacturing process needs to be scalable and reproducible in quality and yield. This is a prerequisite for consistent quality of the final product. The quality of lipids used has a major impact on the performance of the liposomal formulation. Lipids are available in different formats, physical states, and purities from different suppliers, and it is essential to choose the right lipids with the best characteristics depending on the application. When working with any novel lipids, feasibility studies to find the most optimal synthesis route and purification steps, that can be scaled up for GMP production are key.
While the regulatory process for liposome drug products is complicated with many different guidelines from different global authorities, they all agree lipid quality is critical.
To avoid high costs and surprises later in the drug development process, it is important to plan the product development beforehand and work with the same quality of excipients throughout drug development. This highlights the importance of working with the right supplier that offers consistent high-quality products, understands all steps of the drug development process and the regulatory environment and provides a high-level customer support.
Illustrative Blog Post for pharmaexcipients.com – Prepared by Merck KGaA, Darmstadt, Germany by Shiksha Mantri. The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada. — All Rights Reserved
Shiksha Mantri is Global Technical Product Manager for synthetic lipids at Merck KGaA, Darmstadt, Germany, responsible for managing the entire GMP lipids business (portfolio and custom manufacturing). She is also supporting various Merck initiatives as a subject matter expert in several life sciences topics.
Request for more information or a sample of synthetic lipids:
References
- Bulbake, U, et al. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9(2). Web.
- Yingchoncharoen, Phatsapong, Danuta S. Kalinowski and Des R. Richardson. “Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come.” Pharmacological Reviews. 68 701-787 (2016).
- Shrestha, Hina Rajni Bala and Sandeep Arora. “Lipid-Based Drug Delivery Systems.” Journal of Pharmaceutics. 19 May 2014. Web.
- Wagner, A. and K. Vorauer-Uhl. “Liposome technology for industrial purposes.” Drug Deliv. 2011: 591325 (2011).
- Food and Drug Administration (FDA) Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. Apr 2018. Web.
- ICH 11 Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). 1 May 2012. Web.
- “Reflection paper on the data requirements for intravenous liposomal products developed with reference to an innovator liposomal product.” European Medicines Agency (EMA). Feb. 2013. Web.
- Guideline for the Development of Liposome Drug Products. Ministry of Health, Labour and Welfare (MHLW). Mar. 2016. Web.
- ICH Q7 (API GMP): Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Q7. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). 10 Nov. 2014. Web.
- ICH Q1A(R2): Stability Testing of New Drug Substances and Products. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). 6 Feb. 2003. Web.
- ICH Q2(R1): Validation of Analytical Procedures: Text and Methodology. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Nov. 2005. Web.
- Q6A Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). 6 Oct. 1999. Web.
- “The Joint Good Manufacturing Practices Guide for Pharmaceutical Excipients.” International Pharmaceutical Excipients Council (IPEC) and Pharmaceutical Quality Group (PQG). 2017. Web.
- “The Good Distribution Practices Guide for Pharmaceutical Excipients.” International Pharmaceutical Excipients Council (IPEC). 2017. Web..
- Qualification of Excipients for Use in Pharmaceuticals. International Pharmaceutical Excipients Council (IPEC). 2008. Web.
- “The IPEC Excipient Stability Program Guide.” International Pharmaceutical Excipients Council (IPEC). 2010. Web.
- “The IPEC Excipient Composition Guide.” International Pharmaceutical Excipients Council (IPEC). Web.
- “The IPEC Significant Change Guide for Pharmaceutical Excipients.” International Pharmaceutical Excipients Council (IPEC). 2014. Web.
- “IPEC-Americas Excipient Master File Guide.” International Pharmaceutical Excipients Council

