Design, development and in vitro quantification of novel electrosprayed everolimus-loaded Soluplus®/Polyvinyl alcohol nanoparticles via stability-indicating HPLC method in cancer therap
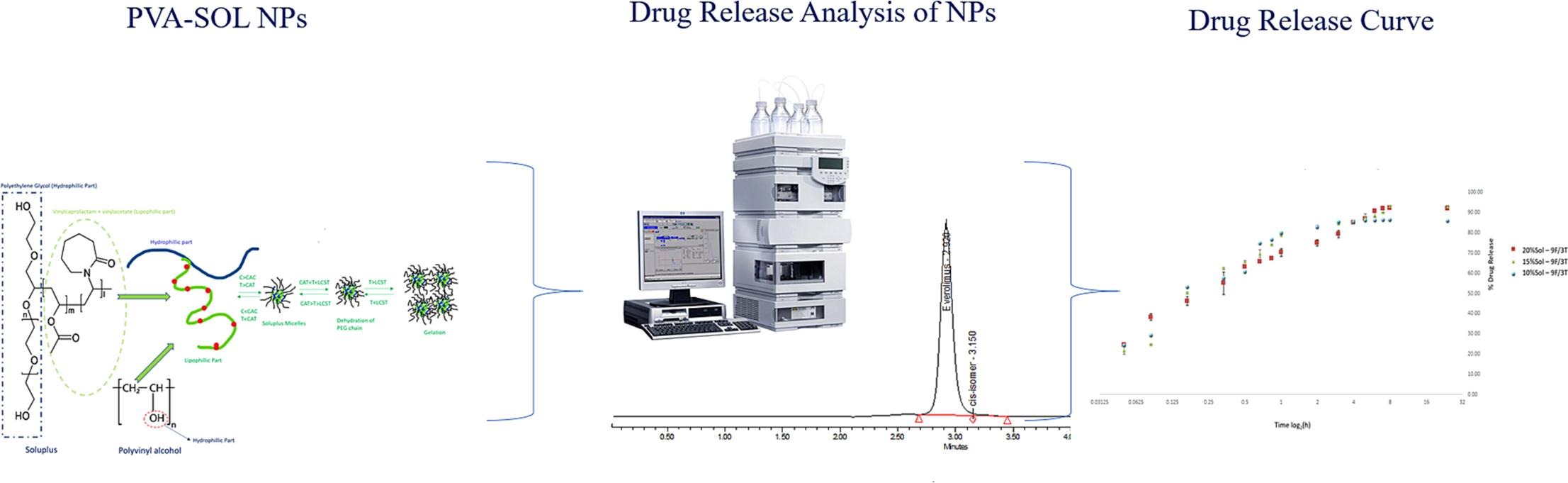
Everolimus (RAD001) a mammalian target of rapamycin has been hampered by poor solubility, affecting its dissolution rate, a relationship that extends to low bioavailability. Nanoparticles (NP) based on Soluplus (SOL®) and Polyvinyl alcohol (PVA) was fabricated by electrospraying (ES) for the delivery of RAD001 to improve anti-tumour efficacy. Electrospraying with established experimental conditions produced PVA-SOL®-RAD001 NP with 71 nm mean diameter, smaller particle size distribution and >90 % encapsulation efficiency. Various polymer-drug concentrations exposed to various freeze–thaw (F/T) cycles were studied for NP optimisation and to enhance its mechanical properties. The optimised NP formulation demonstrated complete encapsulation as well as a sustained and pH dependent drug release profile for in vitro release test.
In addition, to specifically study the degradation profile of RAD001 and to quantify RAD001 in the fabricated NP, a new HPLC method was developed and validated. The purpose and novelty of the HPLC method was also to ensure that RAD001 can be detected at low amounts where other conventional characterisation methods are unable to detect. The developed HPLC method was accurate, precise, robust and sensitive with LOD and LOQ values of 4.149 and 12.575 μg/mL. In conclusion, the novel developed HPLC system can be applied for the quantification of different chemotherapeutic agents and the novel electrosprayed hydrogel NP is a potential drug delivery system to increase solubility and bioavailability of RAD001 in cancer therapy.
Download the full article as PDF here Design, development and in vitro quantification of novel electrosprayed everolimus-loaded Soluplus® Polyvinyl alcohol nanoparticles via stability-indicating HPLC method in cancer therapy
or read it here
Materials
RAD001 (Everolimus) (>99 % purity) was purchased from LC Laboratories (Woburn, MA, USA), Soluplus® was purchased from BTC Europe GmbH -A BASF Group Company, PVA (Mw = 13,000–23,000 g/mol, 87–89 %). The molecular weight of PVA was kept constant at 23, 000 g mol−1. Acetonitrile (CHROMASOLV™, gradient grade, for HPLC, ≥99.9 %) and methanol (CHROMASOLV™, gradient grade, for HPLC, ≥99.9 %) was purchased from Honeywell Research Chemicals, which were used for preparing mobile phase and diluent. De-ionised water was obtained from Milli Q water purification system and met the European Pharmacopeia requirements. The stock and standard solutions were prepared using diluent consisting of (80:20, v/v) methanol: de-ionised water. All other chemical reagents were analytical grade purchased from commercial suppliers.
Lynn Louis, Bor Shin Chee, Marion McAfee, Michael J.D. Nugent, Design, development and in vitro quantification of novel electrosprayed everolimus-loaded Soluplus®/Polyvinyl alcohol nanoparticles via stability-indicating HPLC method in cancer therapy, European Journal of Pharmaceutics and Biopharmaceutics, Volume 191, 2023, Pages 235- 246,ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.09.008.
See the webinar:
“Fast Track development of Biphasic nano-dexamethasone Pellets using galenIQ™”, 12 October 2023:
Get more information & register here for free:


