Fabrication and evaluation of stable amorphous polymer-drug composite particles via a nozzle-free ultrasonic nebulizer
Abstract
We present a promising method for producing amorphous drug particles using a nozzle-free ultrasonic nebulizer with polymers, specifically polyvinylpyrrolidone (PVP), poly(acrylic acid) (PAA), and Eudragit® S 100 (EUD). Model crystalline phase drugs–Empagliflozin, Furosemide, and Ilaprazole–are selected. This technique efficiently produces spherical polymer-drug composite particles and demonstrates enhanced stability against humidity and thermal conditions, compared to the drug-only amorphous particles. The composite particles exhibit improved water dissolution compared to the original crystalline drugs, indicating potential bioavailability enhancements. While there are challenges, including the need for continuous water supply for ultrasonic component cooling, dependency on the solubility of polymers and drugs in volatile organic solvents, and mildly elevated temperatures for solvent evaporation, our method offers significant advantages over traditional approaches. It provides a straightforward, flexible process adaptable to various drug-polymer combinations and consistently yields spherical amorphous solid dispersion (ASD) particles with a narrow size distribution. These attributes make our method a valuable advancement in pharmaceutical drug formulation and delivery.
Introduction
Limited bioavailability of crystalline drugs often arises from their low solubility within the human body, leading to less-than-ideal absorption and therapeutic effectiveness (Bevernage et al., 2013, Boyd et al., 2019). A potential solution is to convert these crystalline drugs into amorphous forms, which can lead to a substantial increase in solubility and bioavailability without compromising therapeutic effects. However, it is important to note that the crystalline state is thermodynamically more stable than the amorphous state (Grohganz et al., 2014, Hancock and Parks, 2000). This often triggers a spontaneous transition from amorphous to crystalline, particularly, under humid conditions, which could significantly affect the drug’s pharmacological and pharmacokinetic properties (Lehmkemper et al., 2017, Onoue et al., 2010). Therefore, it is crucial to manufacture amorphous drugs with kinetically long-lasting stability for use in pharmaceutical applications.
Amorphous solid dispersions (ASDs) present a novel approach to drug formation, aiming to augment the solubility and bioavailability of poorly soluble drugs, commonly found in Biopharmaceutics Classification System (BCS) Class II and IV categories (Bhujbal et al., 2021). In ASDs, drug molecules are dispersed within a polymer matrix in an amorphous state, enhancing solubility and maintaining kinetic stability (Lin et al., 2018). This high-energy amorphous form of ASDs tends to dissolve more efficiently than its crystalline counterpart due to increased internal energy and molecular mobility (Hancock and Zografi, 1997). Additionally, ASDs offer several potential benefits, including their compatibility with both hydrophilic and hydrophobic drugs and applicability across a diverse range of drug-polymer combinations (Singh and Van den Mooter, 2016). Importantly, the intimate dispersion within the polymer matrix in ASDs serves to increase the chemical stability of the drug, providing a layer of protection against environmental factors such as humidity, light, and heat (AboulFotouh et al., 2020, Yu et al., 2018).
Multiple manufacturing techniques have been designed to produce ASDs, including hot-melt extrusion (HME), solvent evaporation methods, and spray drying (Agrawal et al., 2013, Chavan et al., 2020, Simões et al., 2019, Singh and Van den Mooter, 2016). HME, frequently used in pharmaceuticals, involves blending the drug and polymer at elevated temperatures to form a uniform melt, which is then rapidly cooled to induce amorphization (Wilson et al., 2012). Despite its solvent-free nature and compatibility with high throughput production, HME demands the use of heat-stable drugs and costly equipment. On the other hand, solvent evaporation methods offer simplicity and adaptability but might result in inconsistencies due to uneven solvent evaporation (Luebbert et al., 2018). Spray drying is a solvent-based process where a drug-polymer solution is atomized into a heated drying medium, facilitating the rapid formation of solid particles (Patel et al., 2009, Vig and Morgen, 2017). However, this technique poses challenges, particularly the issue of nozzle clogging, which subsequently leads to cleaning complications. More recent developments in this field also include electrospraying and supercritical fluid technology, which have garnered interest due to their potential to circumvent some limitations of traditional techniques. Electrospraying provides superior control over particle size and morphology, but it faces challenges with scalability and nozzle clogging (Nguyen et al., 2016). Supercritical fluid technology is a green, solvent-free approach for ASD production, but it carries high operational costs (Chakravarty et al., 2019, Liu et al., 2020).
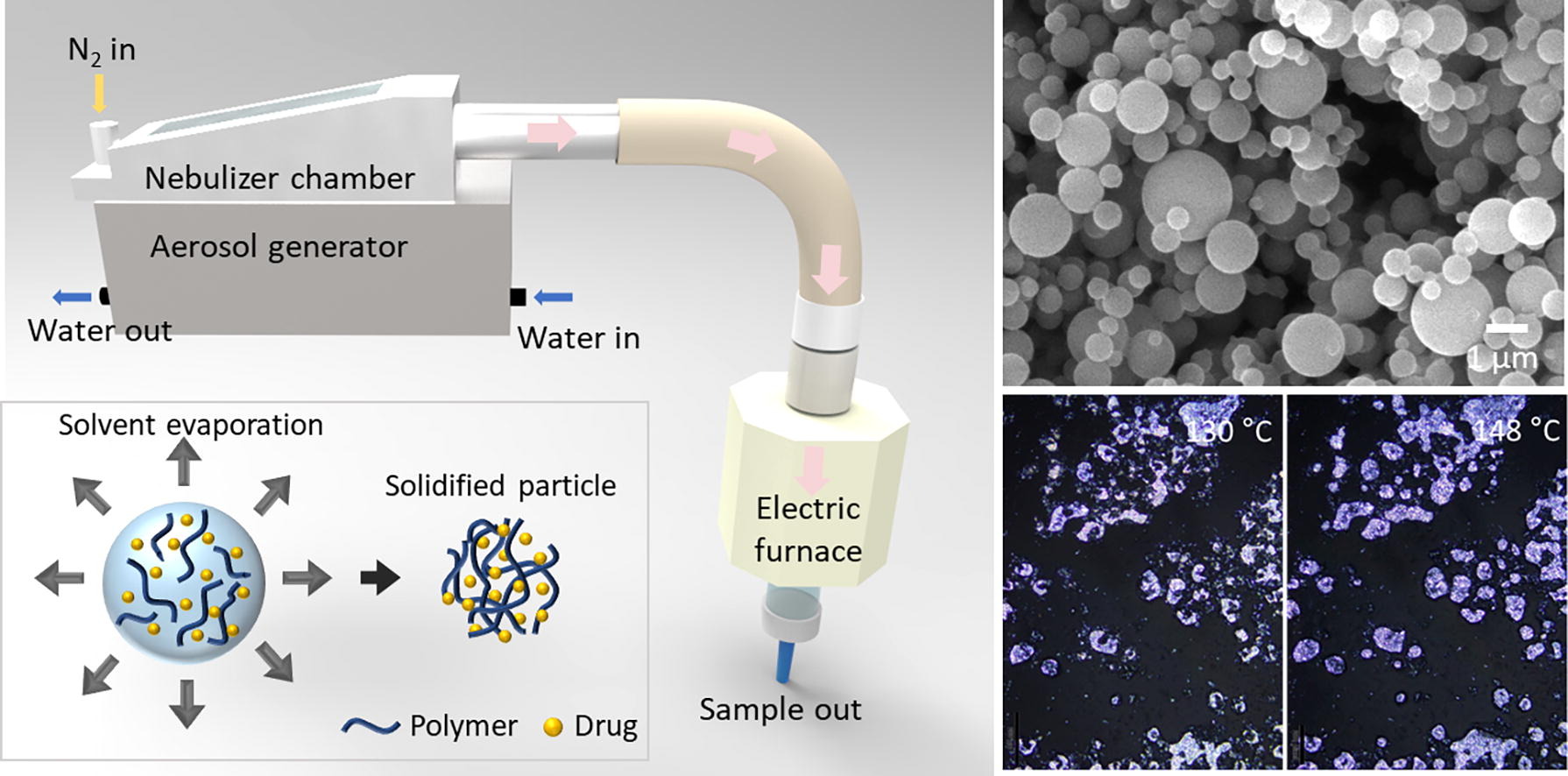
Herein, we introduce a promising approach for producing amorphous polymer-drug composite particles through an ultrasonic nebulizer method that notably eliminates the need for a nozzle. The ultrasonic nebulizer method has previously been utilized to fabricate biocompatible alginate particles used as Pickering stabilizers, spherical polymeric Janus particles, and various metal oxide particles (Daradmare et al., 2020, Daradmare et al., 2022, Im et al., 2017, Im et al., 2020). To the best of our knowledge, this technique remains largely unexplored in the field of ASD production. The nebulizer method employs high-frequency sound waves to produce a mist of tiny solution droplets. Once these droplets are dispersed in air and carried through a pipe channel, they quickly evaporate their solvent content, leading to the formation of solid particles. The absence of a nozzle in this method circumvents prevalent challenges, such as nozzle clogging, while retaining the advantages of a continuous fabrication process operating under mild conditions. It also offers high production efficiency, versatility to accommodate a broad range of materials, moderate uniformity of spherical particle generation, and scalability for both small-scale laboratory work and large-scale industrial production. Note that to apply the ultrasonic nebulizer method for fabricating ASD particles, it is essential that both the polymer and the drug be soluble in a volatile solvent. To demonstrate the versatility of the nebulizer method in ASD particle production, we chose three model crystalline phase drugs—Empagliflozin (EMP), Furosemide (FUR), and Ilaprazole (ILA)—all of which are soluble in methanol, the solvent we employed. EMP is widely used in the treatment of type 2 diabetes (Kohler et al., 2017). FUR serves as a robust diuretic, commonly used for hypertension and edema treatment (Ponto and Schoenwald, 1990). ILA is a highly effective proton pump inhibitor employed primarily in treating gastric acid-related diseases (Cho et al., 2012). We also selected three types of biocompatible and hydrophilic polymers frequently used in lab-scale ASD research, namely polyvinylpyrrolidone (PVP), poly(acrylic acid) (PAA), and Eudragit® S 100 (Eudragit) to further validate our approach. We comprehensively examined the properties of the resulting polymer-drug composite particles, focusing on their amorphous nature, enduring stability against humidity and thermal treatment, as well as their dissolution rate.
Read more here
Materials
Empagliflozin (EMP) and Ilaprazole (ILA) powders were provided by Unicellab, Korea (Table S1). Ilaprazole (ILA) was obtained from Aladdin Biochemical Technology, China. Polyvinylpyrrolidone (PVP, molecular weight (MW) ≈ 29000(29 K), 40000(40 K), and 54000(54 K)) and poly(acrylic acid) (PAA, MW ≈ 1800) were purchased from Sigma–Aldrich, USA. The 29 K PVP was predominantly used unless otherwise specified. Eudragit® S 100 (EUD, MW ≈ 125000) was sourced from Evonik, Germany.
Jieun Lee, Chang Hun Han, In Hwan Oh, Suryanarayana Allu, Hee Jin Kim, Jinsoo Kim, Woo-Sik Kim, Bum Jun Park,
Fabrication and evaluation of stable amorphous polymer-drug composite particles via a nozzle-free ultrasonic nebulizer, International Journal of Pharmaceutics, 2024, 124177, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124177.
Read also our introduction article on Orally Disintegrating Tablets (ODTs) here:


