Fabrication of suppository shells via hot-melt extrusion paired with fused deposition modeling 3D printing techniques
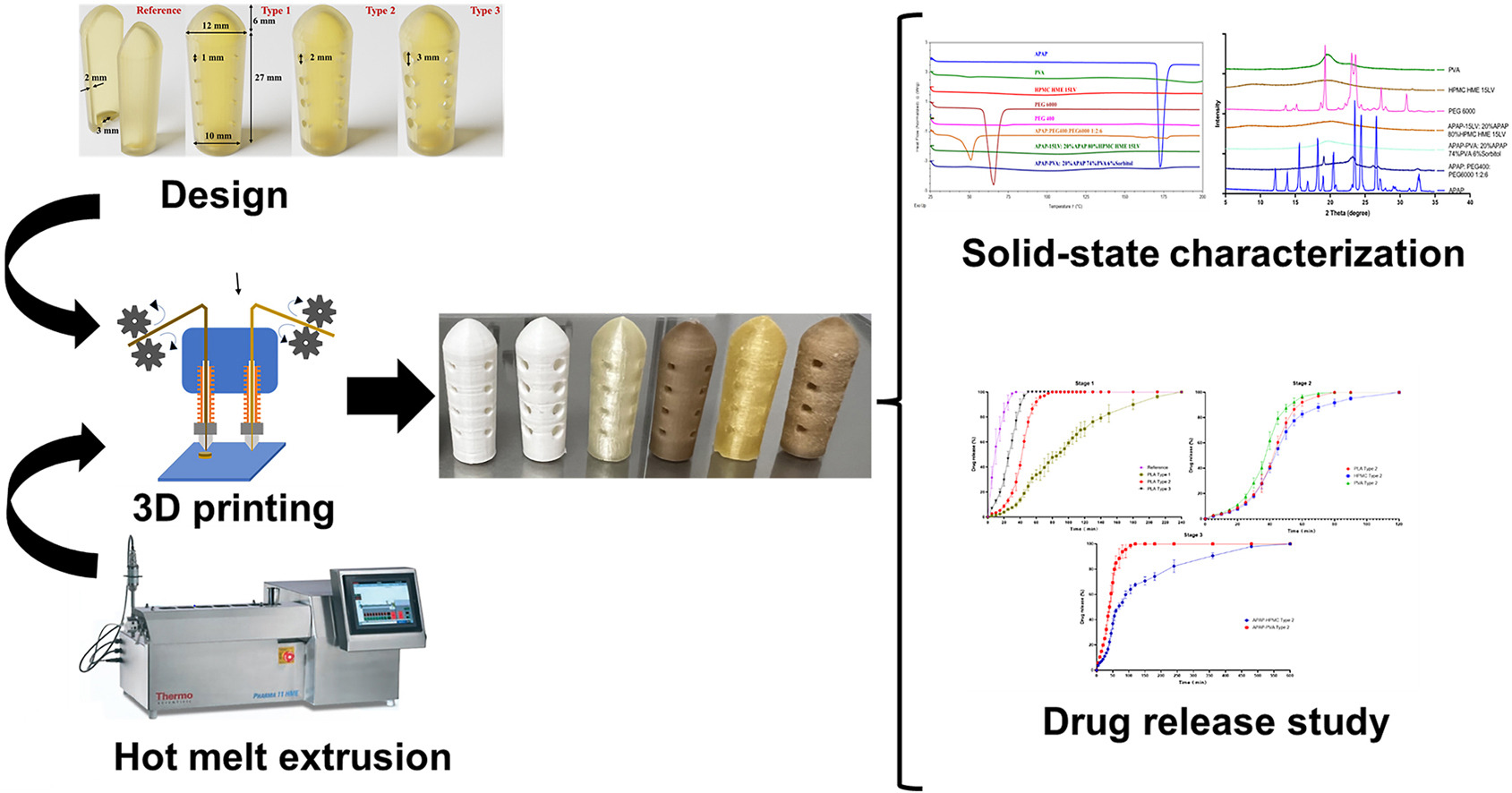
Three-dimensional (3D) printing is a potential technique for developing personalized medicines. Among its applications, mold manufacturing in industrial 3D printing stands out, especially for creating complex structures. This capability has been innovatively extended to drug development. Our study employed fused deposition modeling (FDM) 3D printers to fabricate molds for suppository shells using acetaminophen (APAP), as the model drug, and PVA, PLA, and HPMC HME 15LV as the primary materials.
Highlights
- FDM 3D printing has significant potential in pharmaceutical manufacturing.
- Showcased the flexibility of 3D printing in designing suppository shells.
- The combination of HME and FDM 3D printing is a promising approach in the preparation of personalized medicine and different dosage forms.
This study was segmented into three stages.
- Evaluating the influence of suppository shell pore sizes on drug release
- Distinguishing among different suppository materials
- Formulating a new type of suppository with a drug-containing shell
Our findings demonstrated the ideal pore size (2 mm) for the suppository shell. Furthermore, the release rates varied across the polymers, rank-ordered as PVA > PLA > HPMC HME 15LV. Analyses via powder X-ray diffractometry and differential scanning calorimetry showed that the drug-loaded suppository shells, developed using hot-melt extrusion (HME) and FDM, were amorphous. In contrast, the suppository formulated through fusion revealed some drugs in crystalline state. This study demonstrated the successful and innovative fabrication of suppository shells via HME paired with FDM 3D printing, which can be utilized for personalized medicine.
Read more here
Materials
APAP was purchased from Spectrum Chemical, Inc. (New Brunswick, NJ, USA). Parteck® MXP polyvinyl alcohol (PVA) EMPROVE® ESSENTIAL (molecular weight = 32 kDa) and Parteck® SI 150 (sorbitol) were graciously supplied by EMD Millipore (Burlington, MA, USA). Polyethylene glycol (PEG) 400 and 6000 were purchased from Alfa Aesar, Inc. (Tewksbury, MA, USA). Affinisol™ HPMC HME 15LV (molecular weight = 85 kDa) was donated by Colorcon, Inc. (PA, USA). Polylactic acid (PLA) filament.
Peilun Zhang, Honghe Wang, Sooyeon Chung, Jinghan Li, Sateesh Kumar Vemula, Michael A. Repka, Fabrication of suppository shells via hot-melt extrusion paired with fused deposition modeling 3D printing techniques, Journal of Drug Delivery Science and Technology, 2024, 105491, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105491.
Read also our introduction article on 3D Printing here:


