FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis
This review describes the recently FDA-approved drugs (in the year 2022). Many of these products contain active moieties that FDA had not previously approved, either as a single ingredient or as part of a combination. These products frequently provide important new therapies for patients with multiple unmet diseases. The diverse small molecules are described according to the date of approval and their syntheses is discussed. This review comprises classical chemical scaffolds together with innovative drugs such as a deuterium-containing drug.
Introduction
The constant research for innovative therapies leads every year to molecules that are approved by the United States Food and Drug Administration (FDA), a federal agency of the U.S. Department of Health and Human Services.
Novel building blocks and their connections have been explored much in recent years allowing drug hunters to explore a much bigger chemical space [1].
Small molecule drugs are organic compounds with low molecular weight. For a long time, they have been the backbone of the pharmaceutical industry. There are several benefits that make small molecules important drugs in therapy, such as the possibility of oral administration and cell membrane permeability which allow them to reach specific tissue and targets. For this purpose, small molecule drugs can be designed in order to acquire, for example, specific affinity and selectivity (target, tissue penetration and distribution) [2].
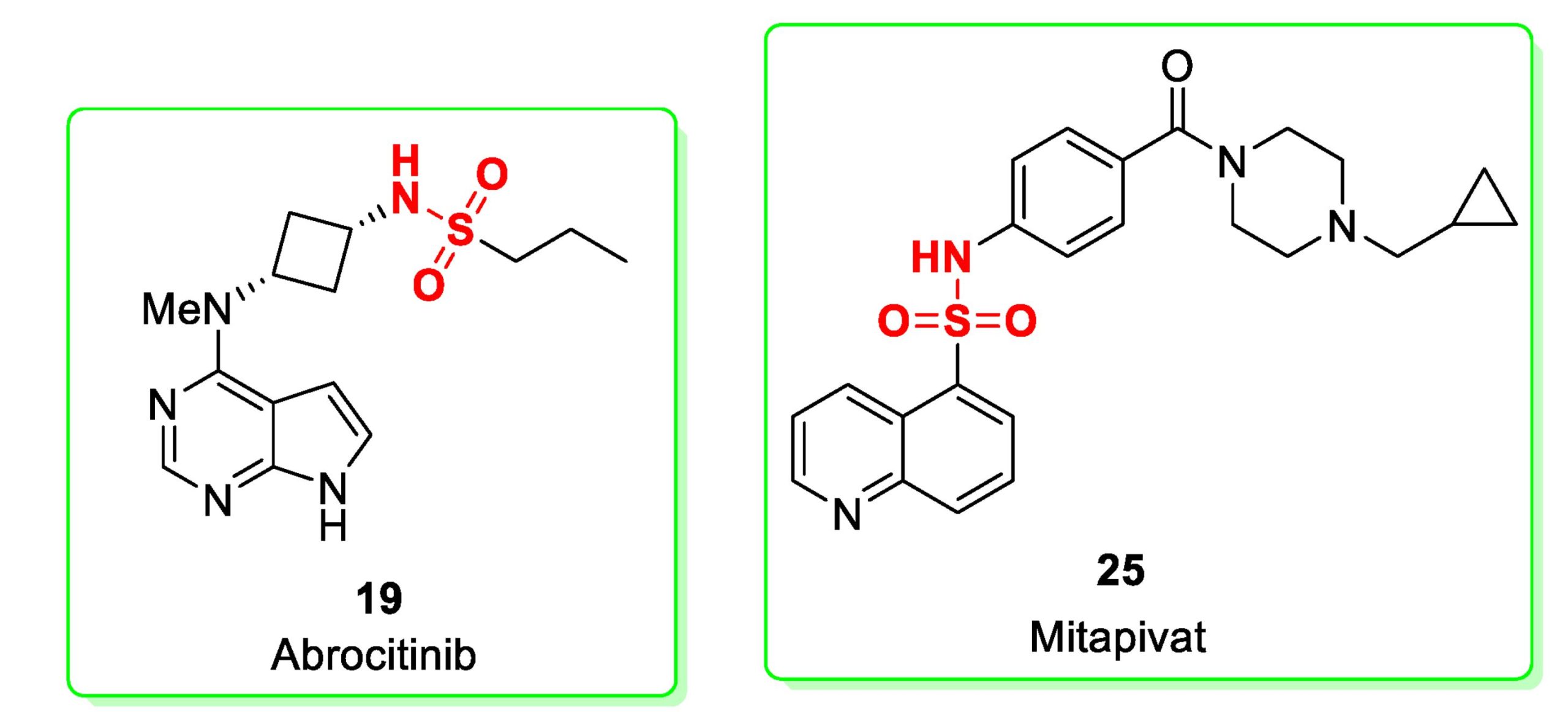
The goal of this review is to highlight the chemical entities approved in the year 2022 for clinical use. The approved drugs (and their synthesis) are listed according to their chronological approval date [3]. This paper focuses specifically on small molecules, as high-molecular weight peptides, vaccines and other biotechnological drugs are beyond the scope of this work. Interestingly, from the perspective of the functional group characterizing the molecules herein discussed, four contain a sulfonamide functionality, one is a macrocycle and two are steroidal structures. Two radioisotope/contrast agents and one deuterium-containing drug were approved. These latter surely represent a very innovative starting point for future drug discovery efforts. Halogenated (mainly chlorinated and fluorinated) drugs still characterize a consistent percentage of the approved drugs, underlining the importance of halogens in drug discovery [4]. All the structures contain at least one aromatic ring except for two drugs which possess steroid-like structure.
Download the full article as PDF here FDA-Approved Small Molecules in 2022_Clinical Uses and Their Synthesis
or read more here
Benedetto Tiz, D.; Bagnoli, L.; Rosati, O.; Marini, F.; Santi, C.; Sancineto, L. FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis. Pharmaceutics 2022, 14, 2538, https://doi.org/10.3390/pharmaceutics14112538

