Fine excipient materials in carrier-based dry powder inhalation formulations: The interplay of particle size and concentration effects
Abstract
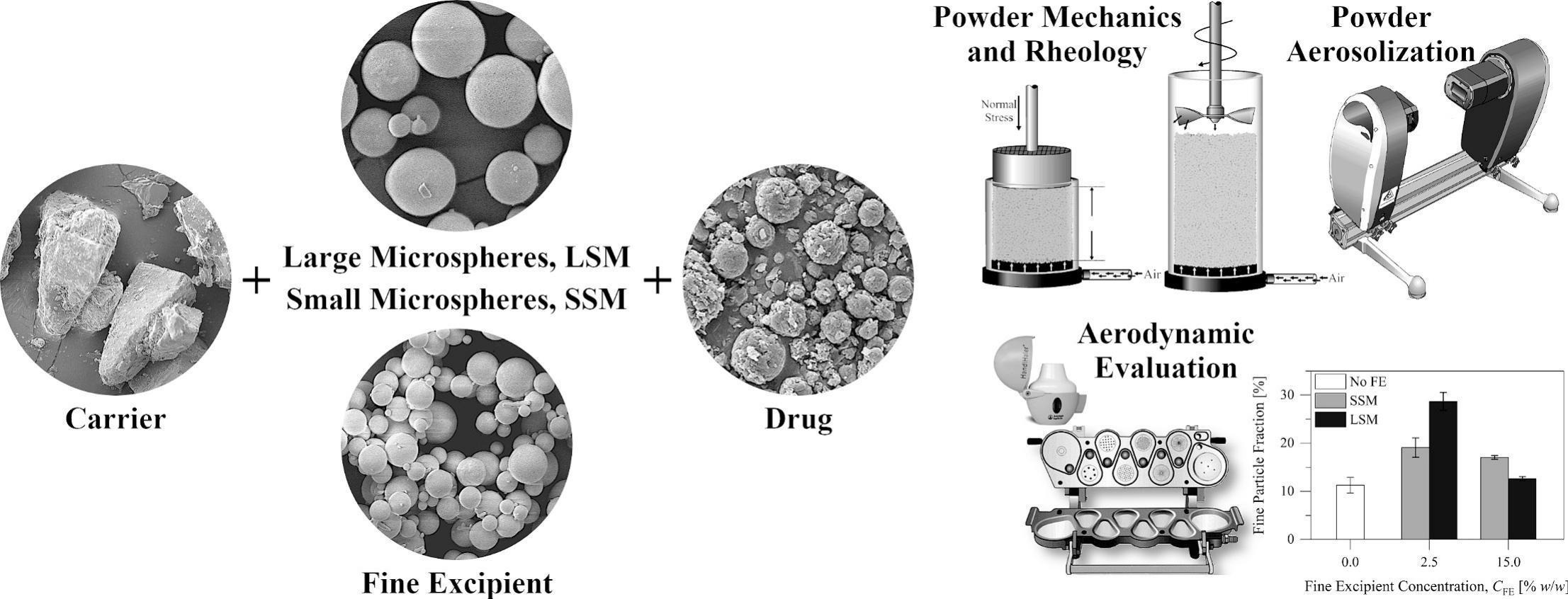
The contributions of fine excipient materials to drug dispersibility from carrier-based dry powder inhalation (DPI) formulations are well acknowledged, although they are not fully elucidated. To improve the understanding of these contributions, we studied the influences of the particle size of the fine excipient materials on various characteristics of carrier-based DPI formulations. We studied two particle size grades of silica microspheres, with volume median diameters of 3.31 μm and 8.14 μm, as fine excipient materials. Inhalation formulations, each composed of a coarse lactose carrier, one of the fine excipient materials (2.5 or 15.0% w/w), and a spray-dried drug (fluticasone propionate) material (1.5% w/w) were prepared. The physical structure, the flow behavior, the aerosolization behavior, and the aerodynamic performance of the formulations were studied. At low concentration, the large silica microspheres had a more beneficial influence on the drug dispersibility than the small silica microspheres. At high concentration, only the small silica microspheres had a beneficial influence on the drug dispersibility. The results reveal diverse influences of fine excipient materials on mixing and dispersion mechanics in carrier-based DPI formulations. At low concentration, the fine particles improved deaggregation and distribution of the drug particles over the surfaces of carrier particles. The large silica microspheres were associated with a greater mixing energy and a greater improvement in the drug dispersibility than the small silica microspheres. At high concentration, the large silica microspheres kneaded the drug particles onto the surfaces of the carrier particles and thus impaired drug dispersibility. As a critical attribute of fine excipient materials in carrier-based dry powder inhalation formulations, the particle size demands robust specification setting.
Introduction
Dry powder inhalers are a keystone in the management of respiratory diseases, such as asthma and chronic obstructive pulmonary disease. Their potential for systemic delivery of drugs and for vaccination is attracting growing interest (de Boer et al., 2017). Most dry powder inhalation (DPI) formulations in the market are carrier-based. A carrier-based DPI formulation is, typically, a ternary blend of a coarse carrier material, a fine excipient material, and a respirable drug material (Elsayed and Shalash, 2018). Typically, coarse carrier particles are 50–200 μm in diameter, whereas fine excipient particles are smaller than 10 μm in diameter. Fine excipient particles are added to promote drug dispersibility from the formulation during inhalation. The complex nature of DPI formulations (Zeng et al., 2001) demands robust specification setting for DPI excipients.
The potential of fine excipient materials to promote drug dispersibility from carrier-based DPI formulations is well acknowledged (Elsayed and Shalash, 2018; Hebbink et al., 2022; Jones and Price, 2006), although it is not fully elucidated. Five principal mechanisms have been proposed to explain this potential. The active site theory (Grasmeijer et al., 2014a; Staniforth, 2000) suggests fine excipient particles fills strongly adhesive or sheltered sites on carrier particles. The agglomerate theory (Adi et al., 2006; Jones et al., 2008; Kinnunen et al., 2015; Lucas et al., 1998) suggests the formation of fine excipient-drug agglomerates, which possess a greater propensity to disperse and deagglomerate than individual drug particles. . The fluidization enforcement hypothesis (Shur et al., 2008) suggests fine excipient particles strengthens dispersion forces generated during aerosolization. The buffer hypothesis (Dickhoff et al., 2006; Grasmeijer et al., 2014b) suggests fine excipient particles larger than drug particles can buffer press-on forces during mixing. The effective deagglomeration hypothesis (Shalash and Elsayed, 2017) suggests fine excipient particles can promote deagglomeration of drug particles during mixing. Arguably, the potential of fine excipient materials to promote drug dispersibility from carrier-based DPI formulations involve joint action of more than one of these mechanisms. The potential depends on characteristics of the inhalation device, the coarse carrier material, and the drug material (Hassoun et al., 2015; Hertel et al., 2018; Muddle et al., 2015).
Attempts were made to define the size of fine excipient particles dominating their contributions to drug dispersibility in carrier-based DPI formulations. Towards this goal, correlations between the concentrations of different size fractions of fine particles and the dispersibility of drug particles from carrier-based DPI formulations were studied. The correlations reported by Guenette et al. (2009) and Sun et al. (2022) suggested that fine excipient particles in carrier-based DPI formulations are best defined as particles smaller than 8.6–12.0 μm in diameter and that these particles similarly, i.e. irrespective of their definite sizes, contribute to drug dispersibility. In contrast, the correlations reported by Handoko et al. (2009) suggested that fine lactose particles with diameters of 5–10 μm are more beneficial than those smaller than 5 μm in diameter. In agreement, Adi et al. (2006) found that a fine lactose material with a volume median particle diameter of 7.9 μm was more beneficial than a fine lactose material with a volume median particle diameter of 3.0 μm. Also in agreement, Grasmeijer et al. (2014b) found that a fine lactose material with a median particle diameter of 3.94 μm (larger than drug particles) was more beneficial than a fine lactose material with a median particle diameter of 1.95 μm (similar in size to drug particles). This could be attributed to the capability of the large fine particles to form relatively more loose agglomerates with drug particles than the small fine particles (Adi et al., 2006) and to the capability of the large fine particles to buffer press-on forces during mixing, thereby weakening adhesion of drug particles to carrier particles (Dickhoff et al., 2006).
The aim of the current study was to improve the understanding of interactions governing the aerodynamic performance of carrier-based DPI formulations. For this purpose, we studied the influences of the particle size of fine excipient materials on various characteristics of carrier-based DPI formulations. Two particle size grades of silica microspheres coated with dimethylpolysiloxane were used as fine excipient materials. Silica microspheres were used to ensure narrow particle size distributions and to avoid interference from other particle characteristics, such as the shape and the surface roughness. The influences of the shape and the surface roughness of fine excipient particles on the characteristics and the performance of carrier-based DPI formulations have been recently studied and discussed by Elsayed et al. (2024). It is noteworthy that silica microspheres are not safe for inhalation because of the risk of silicosis. They are used here simply as model fine particles. Excipient blends, each composed of a DPI carrier material (α-lactose monohydrate) and one of the silica materials at a concentration of 2.5 or 15.0% w/w, were prepared. The two concentrations were selected, based on earlier studies (Almansour et al., 2022a; Elsayed et al., 2024), to represent two structural constructs. The low concentration illustrates, mainly, filling of macropores and interstices between the coarse carrier particles by silica microspheres. The high concentration illustrates separation of the coarse carrier particles by silica microspheres. Inhalation formulations were prepared by mixing each excipient blend with an inhalable, spray-dried drug (fluticasone propionate) material. The influences of the two silica materials on the physical structure, the flow behavior, the aerosolization behavior, and the aerodynamic performance of the powder formulations were studied.
Download the full article as PDF here Fine excipient materials in carrier-based dry powder inhalation formulations
or read it here
Materials
The coarse α-lactose monohydrate carrier material Inhalac® 120 was kindly provided by Molkerei MEGGLE Wasserburg GmbH & Co. KG (Wasserburg am Inn, Germany). The coarse carrier material had a volume-weighted median diameter of 134 μm according to the manufacturer. Two particle size grades of silica microspheres coated with dimethylpolysiloxane were purchased from Cospheric LLC, Santa Barbara, California, USA. The two grades of silica microspheres had number-weighted median diameters of 2–4 μm and 4–8 μm according to the manufacturer. Fluticasone propionate was from Jayco Chemical Industries (Maharashtra, India). Inhalable fluticasone propionate particles were prepared from the raw drug material by nano spray drying using a Büchi nano spray dryer B-90 (Büchi Labortechnik AG, Flawil, Switzerland) as described by Almansour et al. (2022a) and Elsayed et al. (2024). Nano Spray drying is explained in detail by Arpagaus et al. (2018) and Almansour et al. (2022b). Hypromellose capsules (Vcaps® DPI Capsules, Size 3) were kindly provided by Capsugel France S.A.S. (Colmar, France).
Mustafa M.A. Elsayed, Iman M. Alfagih, Katrina Brockbank, Fawaz Alheibshy, Alhassan H. Aodah, Raisuddin Ali, Khaled Almansour, Ahmed O. Shalash, Fine excipient materials in carrier-based dry powder inhalation formulations: The interplay of particle size and concentration effects, International Journal of Pharmaceutics: X, 2024, 100251, ISSN 2590-1567, https://doi.org/10.1016/j.ijpx.2024.100251.
Watch our recorded webinar to “Spray Drying” here:


