Impact of Vertical Blender Unit Parameters on Subsequent Process Parameters and Tablet Properties in a Continuous Direct Compression Line
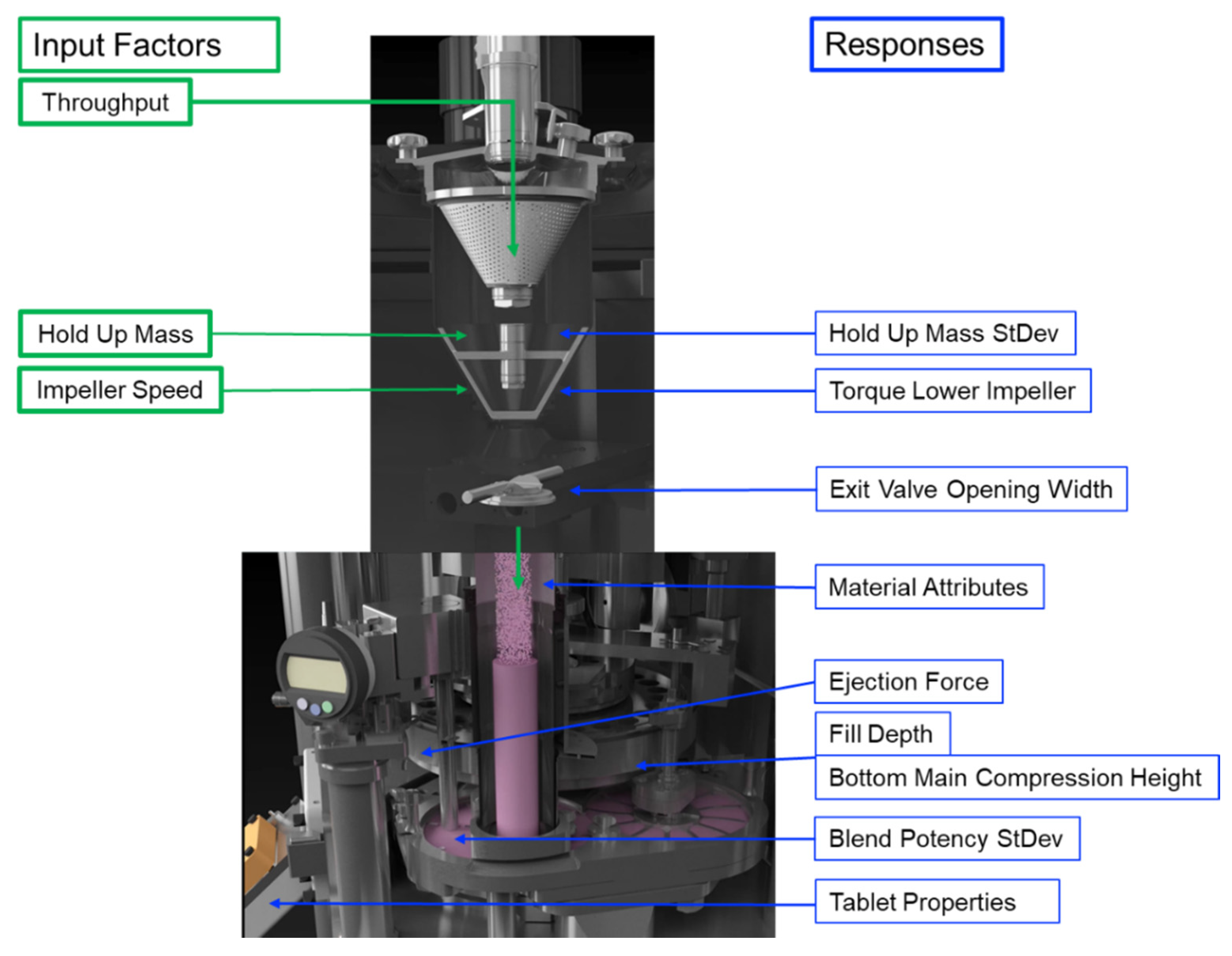
The continuous manufacturing of solid oral-dosage forms represents an emerging technology among the pharmaceutical industry, where several process steps are combined in one production line. As all mixture components, including the lubricant (magnesium stearate), are passing simultaneously through one blender, an impact on the subsequent process steps and critical product properties, such as content uniformity and tablet tensile strength, is to be expected.
A design of experiment (DoE) was performed to investigate the impact of the blender variables hold-up mass (HUM), impeller speed (IMP) and throughput (THR) on the mixing step and the subsequent continuous manufacturing process steps. Significant impacts on the mixing parameters (exit valve opening width (EV), exit valve opening width standard deviation (EV SD), torque of lower impeller (TL), torque of lower impeller SD (TL SD), HUM SD and blend potency SD), material attributes of the blend (conditioned bulk density (CBD), flow rate index (FRI) and particle size (d10 values)), tableting parameters (fill depth (FD), bottom main compression height (BCH) and ejection force (EF)) and tablet properties (tablet thickness (TT), tablet weight (TW) and tensile strength (TS)) could be found.
Furthermore, relations between these process parameters were evaluated to define which process states were caused by which input variables. For example, the mixing parameters were mainly impacted by impeller speed, and material attributes, FD and TS were mainly influenced by variations in total blade passes (TBP). The current work presents a rational methodology to minimize process variability based on the main blender variables hold-up mass, impeller speed and throughput. Moreover, the results facilitated a knowledge-based optimization of the process parameters for optimum product properties.
Download the full article as a PDF here or read it here
Materials: For this trial, saccharin sodium monohydrate (JMC, Ulsan, South Korea), microcrystalline cellulose (Avicel PH 102, FMC, Cork, Ireland), calcium di-phosphate (A-Tab, Innophos, Chicago Heights, IL, USA), sodium starch glycolate (Roquette, Lestrem, France) and magnesium stearate V (Mallinckrodt, St. Louis, MO, USA) were used.
Article information: Kreiser, M.J.; Wabel, C.; Wagner, K.G. Impact of Vertical Blender Unit Parameters on Subsequent Process Parameters and Tablet Properties in a Continuous Direct Compression Line. Pharmaceutics 2022, 14, 278. https://doi.org/10.3390/pharmaceutics14020278


