Long-acting transdermal drug delivery formulations: Current developments and innovative pharmaceutical approaches
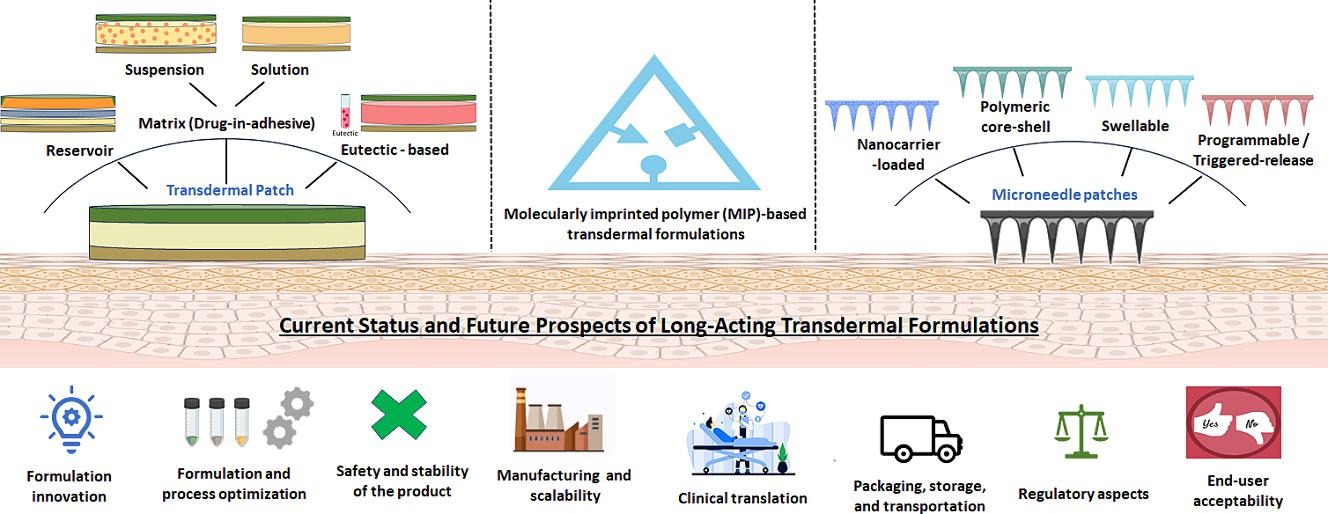
Transdermal administration remains an active research and development area as an alternative route for long-acting drug delivery. It avoids major drawbacks of conventional oral (gastrointestinal side effects, low drug bioavailability, and need for multiple dosing) or parenteral routes (invasiveness, pain, and psychological stress and bio-hazardous waste generated from needles), thereby increasing patient appeal and compliance. This review focuses on the current state of long-acting transdermal drug delivery, including adhesive patches, microneedles, and molecularly imprinted polymeric systems. Each subsection describes an approach including key considerations in formulation development, design, and process parameters with schematics.
An overview of commercially available conventional (adhesive) patches for long-acting drug delivery (longer than 24 h), the reservoir- and matrix-type systems under preclinical evaluation, as well as the advanced transdermal formulations, such as the core-shell, nanoformulations-incorporated and stimuli-responsive microneedles, and 3D-printed and molecularly imprinted polymers that are in development, is also provided. Finally, we elaborated on translational aspects, challenges in patch formulation development, and future directions for the clinical advancement of new long-acting transdermal products.
Table 2: Summary of current literature on long-acting reservoir-type transdermal patches.
| Type of patch | Drug | Indication | Duration of action (model) | Reservoir | Patch specifics | Permeation enhancer |
|---|---|---|---|---|---|---|
| Gel-based | Imipramine hydrochloride[46] | Depression | In vivo delivery up to 30 h (Sprague – Dawley rats) | HPMC (Methocel K4 M) | Release liner (Scotchpak™1022); Porous membrane (CoTran™ 9711); Backing membrane (Scotchpak™ 1009) | 2.5 % (w/v) menthol and 2.5 % (w/v) oleic acid |
| Raloxifene[47] | Breast cancer prevention | In vitro delivery up to 7 days (dermatomed human and porcine skin) | Eudragit®S100 and colloidal silicone dioxide | Release liner (Scotchpak™ 1022) and backing membrane (CoTran™ 9707) | 8.63 % (w/w) and 9.1 % (w/w) oleic acid | |
| Drug powder-based | Zidovudine[52] | HIV/AIDS | In vivo delivery up to 7 days (BALB/c mice) | Lyophilized powder of drug + mannitol | Sterile cylindrical containers (diameter 8 mm and depth 5 mm) | − |
| Lipidic vesicles-loaded | Insulin[48], [49] | Diabetes | In vivo delivery up to 73 h (Sprague – Dawley rats) | Biphasic vesicles in a reservoir | Biphasic vesicles Phase I: soya phosphatidylcholine, cholesterol, propylene glycol and N¬-capryloyl-Ne-lauroyl L-lysine ethyl ester Phase II: linoleamidopropyl-PG-d imonium chloride phosphate and olive oil | − |
| Paroxetine[50] | Depression | In vivo delivery up to 48 h (New Zealand rabbits) | Liposomes of drug in HPMC-E4M reservoir | Liposomes: lecithin phosphatidylcholine and cholesterol | ||
| Nanoparticle-loaded | Repaglinide[51] | Type-2 diabetes | In vivo delivery up to 60 h (Wister rats) | Polymeric nanoparticles of drug in Methocel | Nanoparticles: poly-lactic acid, polycaprolactone, and poloxamer 407 | 10 % (w/w) polyethylene glycol and 1 % dimethyl siloxane |
Download the full article as PDF here Long-acting transdermal drug delivery formulations
or read it here
Tanvi Karve, Amruta Dandekar, Vivek Agrahari, M. Melissa Peet, Ajay K. Banga, Gustavo F. Doncel, Long-acting transdermal drug delivery formulations: Current developments and innovative pharmaceutical approaches,
Advanced Drug Delivery Reviews, Volume 210, 2024, 115326, ISSN 0169-409X, https://doi.org/10.1016/j.addr.2024.115326.
Read more on Parental Excipients here:


