Third-Generation Solid Dispersion Through Lyophilization Enhanced Oral Bioavailability of Resveratrol
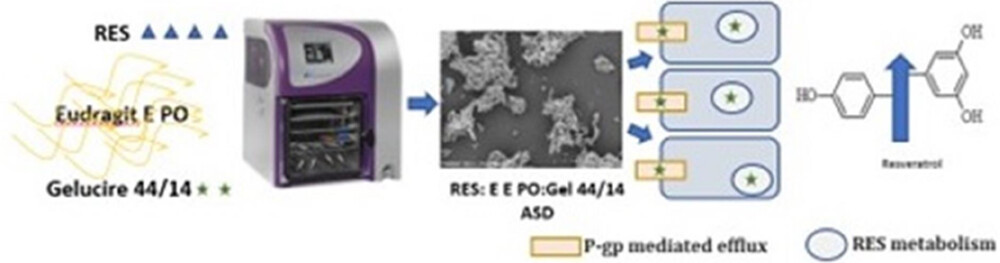
Resveratrol (RES) is a biopharmaceutical classification system (BCS) class II compound with low solubility and high permeability. Several strategies have been explored to overcome the low bioavailability of RES, making the formation of solid dispersions (SDs) one of the most promising. This study aimed at the development of a RES third-generation SD prepared by lyophilization as a strategy to improve RES solubility, dissolution, and oral bioavailability. Eudragit E PO was selected as the hydrophilic carrier in a 1:2 (RES:carrier) ratio, and Gelucire 44/14 as the surfactant, at 16% (w/w) of RES. Differential scanning calorimetry (DSC), scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), polarized light microscopy (PLM), X-ray powder diffraction (XRPD), and particle size distribution (Morphologi 4 Malvern) were used for solid-state characterization and to confirm the conversion of RES to the amorphous state in the SD.
Third-generation SD presented an 8-, 12-, and 8-fold increase of RES solubilized compared to pure RES at pH 1.2, 4.5, and 6.8, respectively, and a 10-fold increase compared to the physical mixture (PM), at pH 6.8, after 24 h. In the gastric environment, the dissolution rate of third-generation SD and PM was similar, and 2-fold higher than pure RES after 30 min, while at pH 6.8, third-generation SD presented approximately a 5-fold increase in comparison to pure RES and PM. Third-generation SD presented higher in vitro intestinal permeability compared to its PM and second-generation SD (without Gelucire 44/14). A 2.4 and 1.7-fold increase of RES permeated, respectively in Caco-2 and Caco-2/HT2-MTX models, was obtained with third-generation SD compared to PM, after 3 h.
Third-generation SD allowed a 3-fold increase of RES bioavailability compared to second-generation SD, after oral administration of 200 mg/kg of RES to Wistar rats. Enhanced RES oral bioavailability was obtained not only by solubility and dissolution improvement, but also by the interference of Gelucire 44/14, with RES metabolism, and inhibition of P-gp-mediated efflux. The presence of excipients like Gelucire 44/14 in the SD allows for greater bioavailability of orally administered RES, making it easier to obtain some of the physiological benefits demonstrated by this molecule.
Download the full article as PDF here: Third-Generation Solid Dispersion Through Lyophilization Enhanced Oral Bioavailability of Resveratrol
or read it here
Excipients mentioned in the article: Eudragit E PO, Compitrol 888 ATO, Docusate sodium, Gelucire 44/14, Polaxamer 407, Tween 80, Labrasol, Cetrimide, sodium dodecyl sulfate (SDS), and Kolliphor RH 40
Hugo Almeida, Bárbara Ferreira, Carlos Fernandes-Lopes, Francisca Araújo, Maria João Bonifácio, Teófilo Vasconcelos, and Bruno Sarmento, Third-Generation Solid Dispersion Through Lyophilization Enhanced Oral Bioavailability of Resveratrol, Cite this: ACS Pharmacol. Transl. Sci. 2024, Publication Date:February 14, 2024
https://doi.org/10.1021/acsptsci.4c00029, © 2024 The Authors. Published by American Chemical Society. This publication is licensed under CC-BY 4.0.
Read also our introduction article on Topical Excipients here:


