Challenges in the Transfer and Scale-Up of Mini-Tableting: Case Study with Losartan Potassium
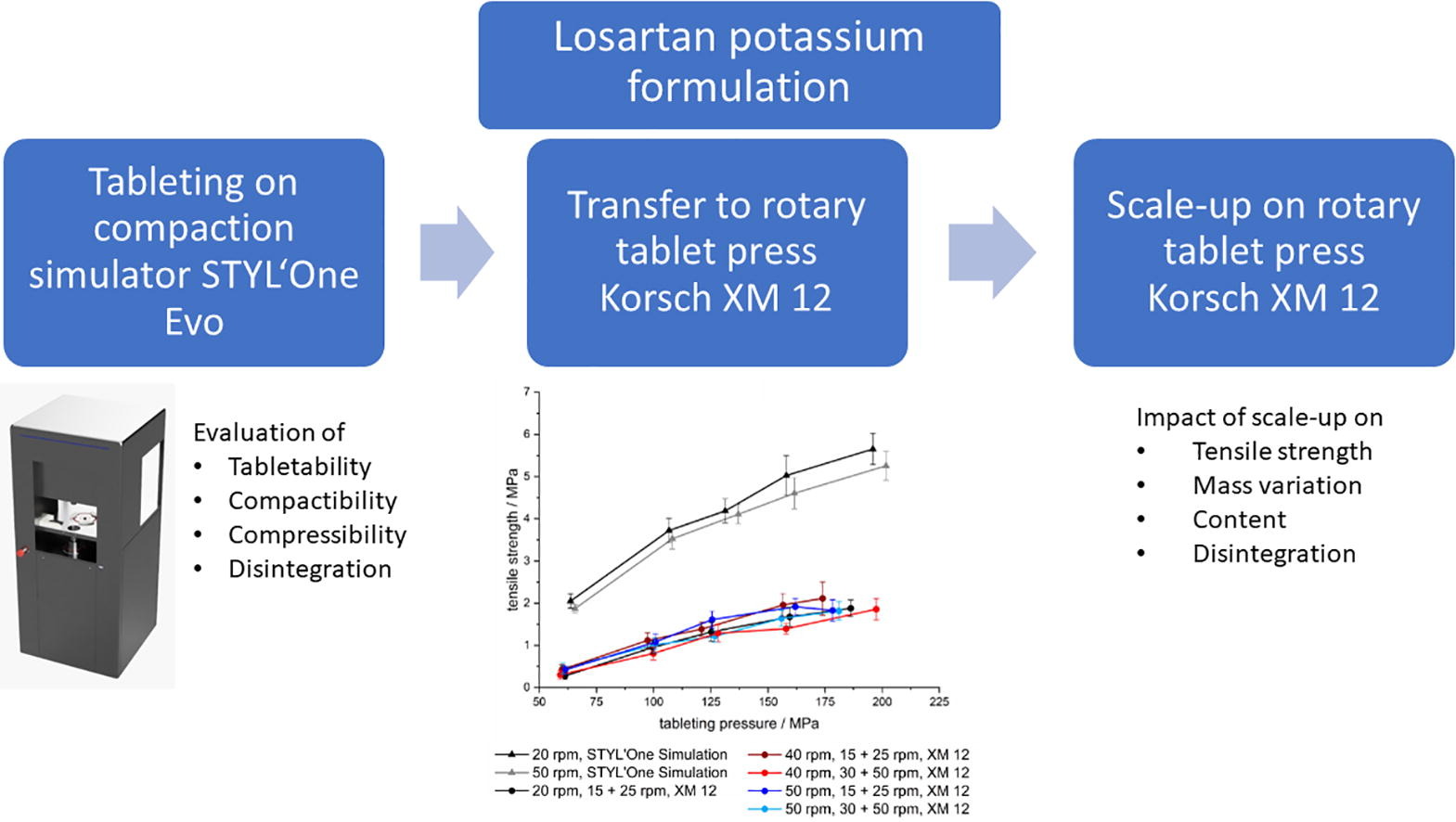
Mini-tablets (MTs) with losartan potassium were developed to treat the rare disease Epidermolysis Bullosa. The focus was placed on transfer and scale-up of a direct compressible formulation from the compaction simulator STYL’One Evo (CS) to the rotary tablet press Korsch XM 12 (RP). Transfer of tabletability and compactibility profiles from CS to RP did not show good agreement, e.g. at a tableting pressure of 125 MPa mean tensile strengths (TS) of 4 MPa on CS and 1-1.5 MPa on RP were reached. These results highlight the impact of the feed frame on final product qualities depending on process and material factors. In the scale-up studies the critical quality attributes (CQAs) mass variation, content uniformity, TS and disintegration time were investigated. After an appropriate run-up time, most CQAs reached a plateau, after reaching a balance between influx, efflux and distribution of lubricant in the feed frame. TS values of 1-2 MPa, disintegration times of max. 50 s, mass variation of 0.9-2.2 % (CV) and acceptance values below 15.0 were reached depending on chosen process parameters.
Introduction
Industrial development and manufacturing of mini-tablets are gaining importance, especially in the field of paediatric medicines following the recommended shift from liquids to oral solid dosage forms, first demanded in a World Health Organization (WHO) report in 2008 and subsequent publications [1], [2], [3]. Additionally, the 2007 EU regulation on medicinal products for paediatric use requiring a Paediatric Investigation Plan (PIP) and supporting changes with Paediatric Use Marketing Authorisation (PUMA) pursues the goal to promote wide availability of child-appropriate medicines [4], [5]. Mini-tablets, have been defined as tablets with a diameter of typically 2-3 mm and a surface-to-volume ratio of at least 2 mm-1 [6], [7]. Mini-tablets have shown good acceptability and swallowability in all subsets of the paediatric population in recent clinical studies [8], [9], [10], [11].
The rare disease Epidermolysis Bullosa (EB) is an inherited skin fragility disorder which can already affect new-borns. The freely water soluble active pharmaceutical ingredient (API) losartan potassium (LP) from the group of sartans was found to be beneficial for the treatment of EB [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Therefore, in a previous study, 2 mm mini-tablets with LP were developed with the focus on a high drug load formulation and taste masking of the bitter API by film-coating [22]. In the present study, LP was continued to be used with a lower dose of 1.4 mg per mini-tablet according to the latest clinical study outcome [15]. The focus was placed on the investigation of the transfer of a direct compressible formulation from the compaction simulator STYL’One Evo (Medelpharm, France) to the industrial rotary tablet press XM 12 (Korsch, Germany) and the scale-up with regard to critical quality attributes (CQAs).
A pivotal advantage of compaction simulators is the opportunity of using small resources, which is especially beneficial in early stages of pharmaceutical development [23]. Various papers are available on research of material properties, tableting processes or formulation development on compaction simulators [7], [22], [24], [25], [26], [27], [28], [29]. Additionally, simulations and transfers to rotary tablet presses [30], [31], [32], [33], [34] and simulation of roll compaction processes [35], [36], [37] have been described.
Scale-up processes are defined as processes of increasing batch sizes with the same manufacturing procedures and formulations. The Food and Drug Administration (FDA) considers a scale-up process for immediate release solid dosage forms a change of batch size of factor 10 using equipments with same design and operating principles among other criteria [38]. Similarly, the European Medical Agency (EMA) notes that pilot batches should be at least 10 % the size of production scale batches and not exceed factor 10 for scale-up steps [39].
A first transfer and scale-up study of 2 mm orodispersible API-free mini-tablets was conducted from the compaction simulator STYL’One Evo to the industrial rotary tablet press XM 12 [31]. It showed the feasibility of the transfer of tabletability and compactibility, but during scale-up CQAs were affected at varying degrees In the present study, transfer and scale-up study from STYL’One Evo to XM 12 should be performed with a newly developed formulation containing LP, accompanied with additional challenges compared to drug-free formulations, specifically the CQAs tensile strength, mass variation, content uniformity and disintegration of 2 mm mini-tablets. In addition, to improve the quality of the manufactured mini-tablets, different process settings and lubrication methods were investigated.
Materials
Losartan potassium (LP) (Dr. Reddy’s Laboratories, Jinnaram Mandal, Telangana, India), silicified microcrystalline cellulose (PROSOLV® SMCC 50 and PROSOLV® SMCC HD 90, JRS Pharma, Rosenberg, Germany) and magnesium stearate (Parteck® LUB MST, Merck, Darmstadt, Germany) were used for the mini-tablet formulation. Methanol (HPLC grade, VWR Chemicals, Radnor, PA, USA), acetonitrile (HPLC grade, Honeywell, Charlotte, NC, USA), phosphoric acid (Merck, Darmstadt, Germany) and monobasic potassium
Read more
Valentinë Lura, Stefan Klinken, Jörg Breitkreutz, Challenges in the Transfer and Scale-Up of Mini-Tableting: Case Study with Losartan Potassium, European Journal of Pharmaceutics and Biopharmaceutics, 2023, , ISSN 0939-6411,
https://doi.org/10.1016/j.ejpb.2023.10.001.
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


